Open Access Article
Open Access ArticleA one pot protocol to convert nitro-arenes into N-aryl amides†
Elisabetta Massolo,
Margherita Pirola,
Alessandra Puglisi,
Sergio Rossi and
Maurizio Benaglia *
*
Dipartimento di Chimica, Università degli Studi di Milano, Via Golgi 19, 20133, Milano, Italy. E-mail: maurizio.benaglia@unimi.it
First published on 23rd January 2020
Abstract
A two-step one pot, experimentally simple protocol, based on readily available and inexpensive reagents allowed the conversion of nitro-arenes directly to N-aryl amides. A metal-free reduction of the nitro group, mediated by trichlorosilane, followed by the addition of an anhydride afforded the corresponding N-aryl carboxyamide, that was isolated after a simple aqueous work up in good-excellent yields. When the methodology was applied to the reaction with γ-butyrolactone, the desired N-aryl butanamide derivative was obtained, featuring a chlorine atom at the γ-position, a functionalized handle that can be used for further synthetic manipulation of the reaction product. Such an intermediate has already been employed as a key advanced precursor of pharmaceutically active compounds.
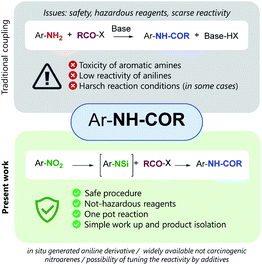
The formation of the amidic bond through the reaction of a carboxylic acid, or its derivative, and an amine is a transformation of outmost importance.1 In 2018, ten years later the first publication, about the ten Key Green Chemistry Research Areas, the six companies and the members of the ACS Green Chemistry Institute® Pharmaceutical Roundtable, confirmed the need for “general methods for catalytic/sustainable (direct) amide or peptide formation”.2 Therefore, the discovery of new methods, possibly catalytic or with improved atom efficiency, is highly desirable, along with the use of environmentally friendly reaction medium and simple isolation processes. When the amide formation involves the use of aromatic amines, the toxicity of anilines becomes also an issue, and safe protocols able to minimize the use of hazardous reagents are needed more than ever.3
Here we report a two-step one-pot, experimentally simple protocol, based on readily available and inexpensive reagents, that allows the conversion of nitro-arenes directly into N-aryl carboxyamide derivatives. The procedure relies on the metal-free reduction of the nitro group by trichlorosilane,3 to in situ generate a N-silylated amine that reacts with an anhydride to afford the expected amide.4 When applied to the reaction with γ-butyrolactone, the method afforded N-aryl butanamides, featuring a chlorine atom at the γ-position, a functionalized handle that may be used to further synthetically manipulate the reaction product.
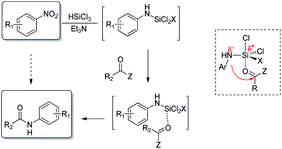
In our study we took advantage of the metal-free reduction of the nitro group to amine5 promoted by trichlorosilane in the presence of tertiary amines. The methodology is highly chemoselective and allows to obtain highly functionalized anilines in good yields after a simple aqueous work up, that hydrolizes the N-silylated amines obtained from the reduction.6 Therefore, we have explored the possibility of exploiting that intermediate and make it react with an anhydride to realize a one-pot synthesis of N-aryl substituted amides starting directly from nitroarene (Fig. 1).
If successful, the proposed strategy would allow to synthesizing amides without direct handling of the highly toxic and carcinogenic aniline derivative, in a single process from relatively inexpensive and widely available nitroarenes. Only few examples are known for the direct conversion of nitroarenes to N-aryl amides, and all of them rely on the use of metal species and often harsh conditions.7 Noteworthy, the present work reports a metal-free approach for the one pot reduction/amidation of nitroarenes under operationally simple conditions.8 We designed a one-pot sequence for N-aryl amides preparation from nitroarenes, trapping the N-silylated reduction intermediate with an acylating agent. The coupling step was supposed to proceed intramolecularly, the silicon center acting as a Lewis acid activated as a hypervalent species (Scheme 1).
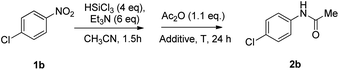
Our investigation started with the study of the acylation of p-chloronitrobenzene with acetic anhydride (Scheme 2). In a typical procedure, to the nitroarene solution in acetonitrile, 6 mol eq. of triethylamine and, then, 4 mol eq. of trichlorosilane were added at 0 °C; the mixture was allowed to warm up to 25 °C in 1.5 hours. Then acetic anhydride and the additive, if needed, were added and, after 24 hours, the reaction was quenched with basic aqueous solution. When the reaction was performed at room temperature, the desired product 2b was obtained in 54% yield; under reflux conditions, the yield was improved up to 70% (entries 1–2 of Table 1). It is worth mentioning that the reaction proceeds also in ethyl acetate, one of the recommended solvents at an industrial level, and afforded the N-(4-chlorophenyl)acetamide in comparable yield.
| Entrya | T (°C) | Additive (mol eq.) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: nitroarene 1 mol eq., triethylamine 6 mol eq., HSiCl3 4 mol eq.b Isolated yields chromatographic purification.c Reaction run at 25 °C in ethylacetate. | |||
| 1 | 25 | — | 54 |
| 2 | 65 | — | 70 |
| 3c | 25 | — | 53 |
| 4 | 25 | (HF)3TEA (3) | 85 |
| 5 | 25 | KF in H2O (10) | 84 |
| 6 | 25 | KF in H2O (4) | 15 |
| 7 | 25 | DMF (6) | 63 |
| 8 | 25 | 2-Pycolinic acid (4) | 80 |
| 9 | 25 | 2-Aminoethanol (2) | 56 |
| 10 | 25 | Methanol (2) | 77 |
| 11 | 25 | Methanol (3) | 83 |
The use of additives was then investigated; it was observed that adding a fluoride anion source, the yield was improved, and increased up to 85% at RT when the commercially available, inexpensive (HF)3TEA was used (entry 4). Fluoride ions are expected to coordinate the silicon atom to generate a penta- or hexacoordinated species that has a strong Lewis acid character and features a partial negative charge on the nitrogen atom.6b,6c This intermediate could therefore coordinate the anhydride, thus leading to a more effective activation of the electrophile, and, at the same time, increasing the nucleophilicity of the nitrogen ligand. Other sources of fluoride anion were less efficient.
An analogously beneficial effect was obtained when Lewis bases as N,N-dimethylformamide were used (entry 7), but typically, large amounts of additives were needed.‡ The addition of methanol was also studied and led to excellent results (entries 10–11, Table 1); § when 3 mol eq. of methanol were added to the reaction mixture, product 2b was isolated in 83% yield, after 24 hours of reaction at room temperature.9
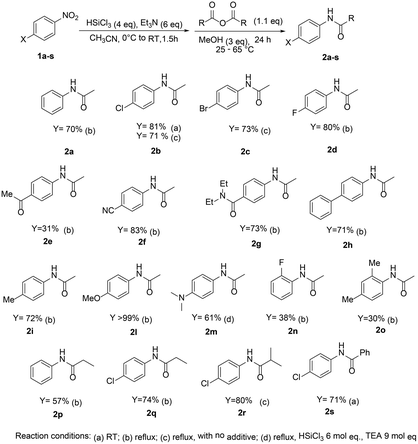
Therefore, the reaction scope was investigated and optimization studies were carried on using methanol as the additive of choice, especially convenient in terms of cost, availability, molecular weight and reduced toxicity, compared to the use of fluoride ion. In Scheme 3 different N-carboxyamide derivatives of anilines, prepared in a one-pot two-step procedure starting from the corresponding nitroarenes are reported.
 | ||
| Scheme 3 Reaction scope of the one pot synthesis of Ar-N-COR 2a–s derivatives starting from nitroarenes and anhydrides. | ||
The protocol afforded good results with nitroarenes featuring electronwithdrawing substituents, when yields higher than 80% were generally observed. The reaction tolerates on the aromatic ring the presence of carbonyl or nitrile groups, which are not affected by the reductive/coupling protocol. However, when 4-methoxynitrobenzene was used, the reaction afforded the amide product 2l in lower yields (30% yield, at 65 °C). No yield improvements were obtained with different additives, but when 3 mol eq. of methanol were added to the mixture, N-4-methoxyphenyl acetamide 2l was obtained, starting from 4-methoxynitrobenzene, in quantitative yield. The protocol proved to be effective also with propionic, benzoic and isobutyric anhydrides, leading to the expected N-aryl amides typically in higher than 70% yields.
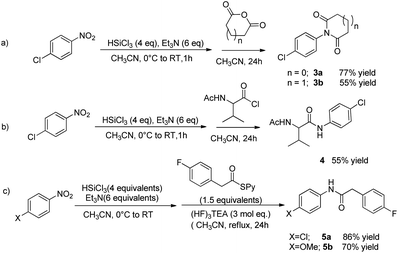
When 4-chloronitrobenzene was reacted with a cyclic anhydrides, the corresponding imide was formed; the reaction with succinic anhydride led to the formation of succinimide 3a in 77% yield, while with glutaric anhydride, the product 3b was isolated in 55% yield (Scheme 4, eqn (a)).
The present procedure works also with acid chlorides; starting from 4-chloronitrobenzene, the reaction with acid chloride of racemic N-acetyl valine afforded the corresponding amide 4 in 55% yield. The one-pot protocol was found to be effective with pyridyl thioesters too and products 5a and 5b were obtained in 86% and 70% yield respectively.¶ The thioesters needed to be added in the reaction mixture after the reduction of the NO2 group was completed, as they were shown to interfere with the reductive step, probably due the coordination of the pyridine moiety to the silicon centre.
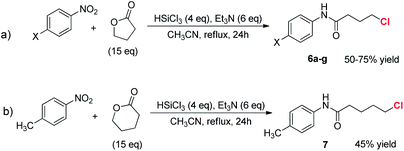
The reaction was also attempted with γ-butyrolactone, a non-activated partner and precursor of a γ-functionalized amide (Scheme 5, eqn (a)).
After a preliminary optimization phase, || it was found that starting from nitrobenzene, running the reaction at 82 °C in the presence of an excess of lactone, the condensation product 6a was obtained in 75% yield, after chromatographic purification (entry 1, Table 2). Interestingly, the obtained product features a chlorine atom at the γ-position, thus offering the opportunity to further functionalize the N-aryl carboxyamide. When the reaction scope was investigated, varying the electronic characteristics of the nitroarene, the procedure was found to work with different substrates, affording the γ-chloro substituted butanamides 6a–e in yields ranging from 50 to 75%.
| Entrya | X | Product | Temp. (°C) | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: to a acetonitrile solution of nitroarene (1 mol eq.), triethylamine (6 mol eq.), and HSiCl3 (4 mol eq.) lactone (5–15 mol eq.) was added.b Isolated yields chromatographic purification. | ||||
| 1 | H | 6a | 82 | 75 |
| 2 | H | 6a | 95 | 67 |
| 3 | Cl | 6b | 82 | 70 |
| 4 | Cl | 6b | 95 | 60 |
| 5 | Br | 6c | 82 | 50 |
| 6 | Me | 6d | 82 | 57 |
| 7 | OMe | 6e | 95 | 51 |
When the 4-nitrotoluene was reacted with δ-valerolactone the expected δ-chloro substituted amide 7 was obtained in 45% isolated yield (eqn (b), Scheme 5).**
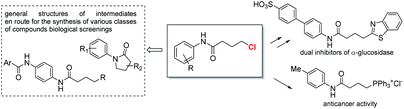
The γ-chloro N-aryl butanamides are versatile building blocks, direct precursor of bioactive products10 and widely exploited intermediates in the preparation of libraries for biological screenings (Fig. 2).11
In conclusion, a two-step one pot, metal-free protocol, based on readily available and inexpensive reagents has been developed to transform directly nitro-arenes into N-aryl amides. When the methodology was applied to the reaction with γ-butyrolactone, the desired N-aryl butanamide was obtained, featuring a chlorine atom at the γ-position, a key intermediate for the preparation of a wide class of biologically active compounds. The proposed strategy allows synthesizing amides without direct handling of the highly toxic and carcinogenic aniline derivatives, in a single, experimentally simple process.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. B. thanks Regione Lombardia for financial support (PON-FESR grant). S. R. thanks Università degli Studi di Milano (grant PSR 2017). E. M. thanks Università degli Studi di Milano for a postdoctoral fellowship.Notes and references
- Reviews: (a) M. T. Sabatini, L. T. Boulton, H. F. Sneddon and T. D. Sheppard, Nat. Catal., 2019, 2, 10–17 CrossRef CAS; (b) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed; (c) J. R. Dunetz, J. Magano and G. A. Weisenburger, Org. Process Res. Dev., 2016, 20, 140–177 CrossRef CAS.
- M. C. Bryan, P. J. Dunn, D. Entwistle, F. Gallou, S. G. Koenig, J. D. Hayler, M. R. Hickey, S. Hughes, M. E. Kopach, G. Moine, P. Richardson, F. Roschangar, A. Steven and F. J. Weiberth, Green Chem., 2018, 20, 5082–5103 RSC.
- Reviews: (a) S. Guizzetti and M. Benaglia, Eur. J. Org. Chem., 2010, 5529–5541 CAS; (b) S. Jones and C. J. A. Warner, Org. Biomol. Chem., 2012, 10, 2189–2200 RSCFor selected, recent contributions not included in previous reviews: (c) L. Chen, C. Wang, L. Zhou and J. Sun, Adv. Synth. Catal., 2014, 356, 2224–2230 CrossRef CAS; (d) X.-Y. Hu, M.-M. Zhang, C. Shu, Y.-H. Zhang, L.-H. Liao, W.-C. Yuan and X.-M. Zhang, Adv. Synth. Catal., 2014, 356, 3539–3544 CAS; (e) C. Wang, X. Wu, L. Zhou and J. Sun, Org. Biomol. Chem., 2015, 13, 577–582 RSC; (f) J. Ye, C. Wang, L. Chen, X. Wu, L. Zhou and J. Sun, Adv. Synth. Catal., 2016, 358, 1042–1048 CrossRef CAS; (g) A. Chelouan, R. Recio, L. G. Borrego, E. Álvarez, N. Khiar and I. Fernandez, Org. Lett., 2016, 18, 3258–3261 CrossRef CAS PubMedFor pioneer works with aminoacid-derived catalysts see: (h) A. V. Malkov, A. Mariani, K. N. MacDougal and P. Kočovský, Org. Lett., 2004, 6, 2253–2256 CrossRef CAS PubMed; (i) A. V. Malkov, A. J. P. S. Liddon, P. Ramírez-López, L. Bendová, D. Haigh and P. Kočovský, Angew. Chem., Int. Ed., 2006, 45, 1432–1435 CrossRef CAS PubMed; (j) S. Guizzetti, M. Benaglia and S. Rossi, Org. Lett., 2009, 11, 2928–2931 CrossRef CAS PubMed; (k) Y. Jiang, X. Chen, Y. Zheng, Z. Xue, C. Shu, W. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2011, 50, 7304–7307 CrossRef CAS PubMed; (l) Y. Jiang, X. Chen, X. Hu, C. Shu, Y. Zhang, Y. Zheng, C. Lian, W. Yuan and X. Zhang, Adv. Synth. Catal., 2013, 355, 1931–1936 CrossRef CAS; (m) A. Genoni, M. Benaglia, E. Massolo and S. Rossi, Chem. Commun., 2013, 49, 8365–8367 RSC; (n) P. C. Barrulas, A. Genoni, M. Benaglia and A. J. Burke, Eur. J. Org. Chem., 2014, 7339–7442 CrossRef CAS; (o) D. Brenna, R. Porta, E. Massolo, L. Raimondi and M. Benaglia, ChemCatChem, 2017, 9, 941–945 CrossRef CAS.
- N. Tao, G. Liu, L. Bai, L. Tang and C. Guo, Chemosphere, 2017, 169, 467–473 CrossRef CAS PubMed.
- M. Orlandi, F. Tosi, M. Bonsignore and M. Benaglia, Org. Lett., 2015, 17, 3941–3943 CrossRef CAS PubMed Review: M. Orlandi, D. Brenna, R. Harms, S. Jost and M. Benaglia, Org. Process Res. Dev., 2018, 22, 430–434 CrossRef.
- (a) M. Orlandi, M. Benaglia, F. Tosi, R. Annunziata and F. Cozzi, J. Org. Chem., 2016, 81, 3037–3041 CrossRef CAS PubMedFor reviews on hypervalent silicon species see: (b) M. Benaglia, S. Guizzetti and S. Rossi, Silicate-Mediated Stereoselective Reactions Catalyzed by Chiral Lewis Bases, in Catalytic Methods in Asymmetric Synthesis, ed. John Wiley & Sons, Inc., 2011, pp. 579–624 Search PubMed; (c) S. Rossi and S. E. Denmark, Lewis Base-Catalyzed, Lewis Acid-Mediated Reactions (n → σ*), in Lewis Base Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2016, pp. 1039–1076 Search PubMed.
- (a) V. Kumar, M. Kumar, S. Sharma and N. Kumar, RSC Adv., 2014, 4, 11826 RSC; (b) C. W. Cheung, M. Leendert Ploeger and X. Hu, Nat. Commun., 2017, 8, 14178 CrossRef PubMed; (c) C. W. Cheung, J.-A. Ma and X. Hu, J. Am. Chem. Soc., 2018, 140, 6789 CrossRef CAS PubMed; (d) F. Zhou, D.-S. Wang, X. Guan and T. G. Driver, Angew. Chem., Int. Ed., 2017, 56, 4530 CrossRef CAS PubMed; (e) C. W. Cheung, M. Leendert Ploeger and X. Hu, ACS Catal., 2017, 7, 7092 CrossRef CAS; (f) C. W. Cheung, N. Shen, S.-P. Wang, A. Ullah, X. Hu and J.-A. Ma, Org. Chem. Front., 2019, 6, 756 RSC.
- For a pioneering example of metal-free synthesis of N-aryl amides see: (a) W. Guo, J. E. Gomez, L. Martinez-Rodriguez, N. A. G. Bandeira, C. Bo and A. W. Kleij, ChemSusChem, 2017, 10, 1969 CrossRef CAS PubMedFor a very recent contribution see also: (b) T. W. Bousfield, K. P. R. Pearce, S. B. Nyamini, A. A. Dimakis and J. E. Camp, Green Chem., 2019, 21, 3675 RSCSee also: (c) Y. F. Sun, G. H. Xu, M. J. Gao, Q. L. Hu and J. J. Ma, Curr. Org. Chem., 2017, 21, 2884–2889 CAS; (d) M. A. Kakroudi, F. Kazemi and B. Kaboudin, RSC Adv., 2014, 4, 52762–52768 RSC.
- The use of tetramethoxysilane as reagent able to promote the acid-amine coupling to form amides has been recently described, see: D. C. Braddock, P. D. Lickiss, B. C. Rowley, D. Pugh, T. Purnomo, G. Santhakumar and S. J. Fussell, Org. Lett., 2018, 20, 950–953 CrossRef CAS PubMed.
- M.-Y. Wang, X.-C. Cheng, X.-B. Chen, Y. Li, L.-L. Zang, Y.-Q. Duan, M.-Z. Chen, P. Yu, H. Sun and R.-L. Wang, Chem. Biol. Drug Des., 2018, 92, 1647–1656 CrossRef CAS PubMed.
- (a) W. C. Drewe, R. Nanjunda, M. Gunaratnam, M. Beltran, G. N. Parkinson, A. P. Reszka, W. D. Wilson and S. Neidle, J. Med. Chem., 2008, 51, 7751–7767 CrossRef CAS PubMed; (b) F. Cuenca, M. J. B. Moore, K. Johnson, B. Guyen, A. De Cian and S. Neidle, Bioorg. Med. Chem. Lett., 2009, 19, 5109–5113 CrossRef CAS PubMed; (c) S. Sparapani, S. M. Haider, F. Doria, M. Gunaratnam and S. Neidle, J. Am. Chem. Soc., 2010, 132, 12263–12272 CrossRef CAS PubMed; (d) Y.-H. Huang, S.-R. Wang, D.-P. Wu and P.-Q. Huang, Org. Lett., 2019, 21, 1681–1685 CrossRef CAS PubMed; (e) L.-H. Li, Z.-J. Niu and Y.-M. Liang, Chem. - Eur. J., 2017, 23, 15300–15304 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10758d |
| ‡ Reaction yield was further improved, up to 73%, but the addition of 20 mol eq. of DMF was necessary. |
| § The role of methanol and the nature of its interaction with the N-silylated amine intermediate is under investigation. The formation of (MeO)nSi–N–Ar species might be envisaged, but further studies are needed to clarify the point. |
| ¶ Under the present reaction conditions, alkyl or benzyl esters do not react; further studies are underway to overcome that reagent limitation. Arylthioesters afforded the product in moderate yields. |
| || For further details on preliminary investigation and optimization studies of parameters, such as reagents stoichiometry, temperature, solvent and other experimental details see the ESI.† |
| ** The reaction between 4-methylnitrobenzene and β-propiolactone afforded the corresponding amide in low yields (up to 23% yield); further studies, involving also other 4–7 membered lactones are underway. |
| This journal is © The Royal Society of Chemistry 2020 |