Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: The extract from the roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils attenuates DSS-induced ulcerative colitis by regulating the Nrf2/NF-κB signaling pathways†
Xinyu Li‡
ab,
Rui Liu‡a,
Yanmin Zhaoab,
Naying Gaobc,
Xin Jinb,
Xiaoxia Gaoc,
Tan Li*b and
Dailin Liu *ab
*ab
aTianjin University of Traditional Chinese Medicine, Tianjin 300193, China
bTianjin Key Laboratory for Prevention and Control of Occupational and Environmental Hazard, Logistics University of Chinese People's Armed Police Force, Tianjin 300309, P. R. China. E-mail: dailinlch@163.com; Tanli20042001@163.com; Fax: +86-22-84876608; Fax: +86-22-84876589; Tel: +86-22-84876608 Tel: +86-22-84876589
cDepartment of Traditional Chinese Medicine, Guangdong Pharmaceutical College, Guangdong 510006, P. R. China
First published on 4th March 2020
Abstract
Ulcerative colitis (UC) is a chronic non-specific inflammatory bowel disease. Many natural medicines or ethnic drugs have been proven to be effective and beneficial in the treatment of UC. Among these, the root of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils. (ROS), a kind of Yi nationality medicine called “GU-GONG-GUO,” has been shown to be useful as a therapeutic agent for sputum and diarrhea. However, the effects and mechanisms of ROS extract (ROE) on UC remain relatively unknown. The aim of this study was to explore the protective effects and the underlying mechanisms of ROE on UC. First, we established a dextran sulfate sodium (DSS)-induced experimental colitis mouse model and then evaluated the anti-inflammatory effects of ROE in vivo. Then, RAW264.7 cells were stimulated with lipopolysaccharide (LPS) in the presence or absence of ROE to explore its mechanisms in vitro. It was found that ROE attenuated DSS-induced colon length shortening, body weight loss, and colonic pathological damage. Furthermore, ROE inhibited the activity of nitric oxide synthase (NOS), myeloperoxidase (MPO), and malondialdehyde (MDA). Also, ROE decreased several LPS-induced inflammatory cytokines including TNF-α, IL-6, and IL-1β in RAW264.7. In addition, ROE inhibited the expression of p-NF-κB, p-IKKα/β, and Keap1 proteins and increased the expression of Nrf2 and HO-1 proteins. Our study suggested that ROE prevented DSS-induced colitis though the Nrf2/NF-κB pathway. Overall, it can be concluded that ROE may be a promising and effective agent for UC.
Introduction
Ulcerative colitis (UC) is a chronic debilitating disease characterized by relapsing intestinal inflammation and ulcers with no available cure. Its pathogenic factors are complex and easily cancerous, and its prevalence rate is almost 250 cases per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons and is increasing worldwide in individuals of all ages.1 When this disease attacks, the clinical symptoms shown include diarrhea, abdominal pain, constipation, abdominal distension, and weight loss. In addition, patients often suffer from anemia, skin and mucous membrane injuries.2 The pathogenesis of UC is not fully elucidated, although immune, environmental, and genetic factors, including the microbiome, are thought to be of importance. Immune function abnormalities and their interactions with commensal microbiota in the digestive tract are the critical factors that promote the development of UC.3 Numerous studies have suggested that tumor necrosis factor (TNF)-α and interleukins (ILs) play central roles in modulating the inflammation response of UC. Moreover, the occurrence of UC is a multi-gene participation process involving multiple cell signal pathways, including nuclear factor κB (NF-κB), mitogenactivated protein (MAPK), and toll-like receptors 4 (TLR4); among these, NF-κB, a key transcription factor, promotes the transcription of the gene encoding proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which are considered to be related to the development and pathogenesis of UC.4–6 Furthermore, Nrf2 is a classical anti-oxidative and anti-inflammatory protein and is closely implicated with the occurrence of UC.7 It has been reported that the protein expression of Nrf2 in epithelial cells increases in patients suffering from UC.8 Thus, Nrf2 and NF-κB signaling pathways are closely related to the progression of UC.9
000 persons and is increasing worldwide in individuals of all ages.1 When this disease attacks, the clinical symptoms shown include diarrhea, abdominal pain, constipation, abdominal distension, and weight loss. In addition, patients often suffer from anemia, skin and mucous membrane injuries.2 The pathogenesis of UC is not fully elucidated, although immune, environmental, and genetic factors, including the microbiome, are thought to be of importance. Immune function abnormalities and their interactions with commensal microbiota in the digestive tract are the critical factors that promote the development of UC.3 Numerous studies have suggested that tumor necrosis factor (TNF)-α and interleukins (ILs) play central roles in modulating the inflammation response of UC. Moreover, the occurrence of UC is a multi-gene participation process involving multiple cell signal pathways, including nuclear factor κB (NF-κB), mitogenactivated protein (MAPK), and toll-like receptors 4 (TLR4); among these, NF-κB, a key transcription factor, promotes the transcription of the gene encoding proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which are considered to be related to the development and pathogenesis of UC.4–6 Furthermore, Nrf2 is a classical anti-oxidative and anti-inflammatory protein and is closely implicated with the occurrence of UC.7 It has been reported that the protein expression of Nrf2 in epithelial cells increases in patients suffering from UC.8 Thus, Nrf2 and NF-κB signaling pathways are closely related to the progression of UC.9
The most current standard therapy for UC includes the use of aminosalicylates (sulphasalazine and mesalamine), glucocorticosteroids (prednisolone and budesonide), immunosuppressants (azathioprine, 6 mercaptopurine, and methotrexate), and biologic agents (infliximab and adalimumab).10,11 However, these treatments can cause severe side effects, including diarrhea, cramps, abdominal pain accompanied by fever, and high blood pressure in the context of continued administration.12 Many researchers are now turning to natural Chinese medicine in their search for effective components that can be used against UC.
Traditional Chinese Medicine (TCM) has a long history of treating UC. The Chinese traditional medical terms for UC, called “da xia xie”, mean long-term dysentery, intestinal obstruction, and diarrhea. According to the traditional Chinese medical theory, patients with acute UC mainly have excess dampness due to the diminished function of the spleen. Treatment using TCM to invigorate the spleen and dispel dampness can help relieve abdominal pain, diarrhea, and inflammation. Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils is a kind of Yi nationality medicine, which has the effects of stopping diarrhea and an astringent intestinal tract and could be used to treat colitis for years. At the same time, it is the main ingredient in the Chinese patent medicine “Changshu Tablet,” which is used for intestinal diseases, such as diarrhea and bacillary dysentery. In our previous study,13 we found that the extract from the roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils (ROE) could inhibit the contraction of isolated intestinal smooth muscle induced by acetylcholine stimulation. Moreover, our pre-experimental results also showed that ROE had a certain inhibitory effect on an experimental UC animal model.
In this present study, the anti-inflammatory mechanisms of ROE were further investigated using a DSS-induced UC mouse model and RAW264.7 cells stimulated by LPS both in vivo and in vitro. Moreover, the mechanisms of the ROE based on the Nrf2/NF-κB pathway were explored further. Our results demonstrated that ROE exhibited effective protection for UC via regulating the Nrf2/NF-κB signaling pathways. In conclusion, our results lay down a theoretical foundation for the comprehensive utilization of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils.
Materials and methods
Chemicals and reagents
LPS (batch number: 017M4112V) was obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). NO, NOS, MDA, and SOD activity assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ELISA kits for mouse TNF-α, IL-6, and IL-1β were purchased from Beijing 4A Biotech (4A Biotech Co. Ltd., Beijing, China). Nrf2 (catalog no. 12721), HO-1 (catalog no. 70081), Keap1 (catalog no. 4617), phospho-NF-κB-p65 (Ser536, catalog no. 3033), and phospho-IKKα/β (Ser176/180, catalog no. 2697) were purchased from Cell Signaling Technology (Danvers, MA, USA). Sulfasalazine enteric-coated tablets (SSZ, positive drug) were purchased from Shanghai Fuda Pharmaceutical (Fuda Pharmaceutical Co. Ltd., Shanghai, China). DSS was purchased from MP Biomedicals, USA. BAY 117082 (NF-κB Inhibitor, Lot 17893) was purchased from MedChemExpress USA.Plant materials and extract preparation
Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils, medicinal materials were collected from Qujing City of Yunnan Province. It was identified as the roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils by Professor Chen Yu, an expert in plant taxonomy, Kunming Institute of Botany, Chinese Academy of Sciences. Specimens were deposited in the Department of Pharmacognosy and Pharmaceutics, Armed Police Logistics College (WJL-012). The dried roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils (5 kg), cut into small pieces of 2 cm square, were extracted twice by reflux with a 6-fold volume 60% (v/v) ethanol solution for 2 h. After filtration, the collected extract was subsequently evaporated to semi-dryness using a rotary vacuum evaporator (EYELA N-1100S, Japan) at 45 °C. Then, the residue was combined to obtain a crude extract, which was concentrated under reduced pressure to ethanol, and then partitioned to 5 L with water. It was further dried under reducing press to obtain a dry extract (ROE, 52 g, batch number 20150525). The contents of total tannin and total triterpenoids in the ROE were determined using the casein method14 and vanillic aldehyde-glacial acetic acid method,15 and they were found to be 47.5% and 26.3%, respectively.HPLC analysis
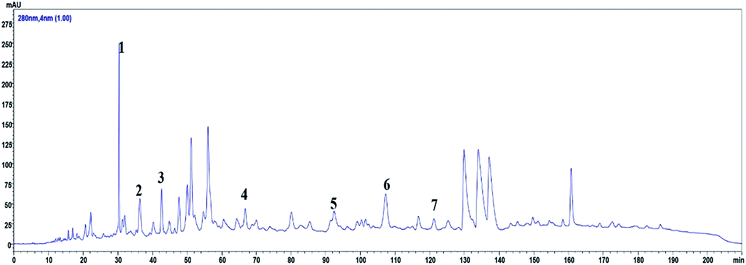
The main polyphenols in ROE were determined using high-pressure liquid chromatography (HPLC, SHIMAZU LC-20AT liquid chromatography, Japan) with a photodiode array (PDA, SHIMAZU SPD-M20A) detector. Separation and identification were based on our previous report16 and performed with a COSMOSIL 5C18-MS-II (4.6 mm × 250 mm, 5 μm) at 30 °C with a gradient elution solution A, composed of a formic acid–water solution (0.1% formic acid), and solution B, comprising an acetonitrile and formic acid–water solution (0.1% formic acid) (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; v/v), which were delivered at a flow rate of 0.7 mL min−1 as follows: 0 min, 96% (A); 180 min, 70% (A); 205 min, 96% (A); and 210 min, 0% (A). The UV spectra of every identified peak were recorded at 280 nm. The tested standards, including procyanidin B3 (1), EGC (2), catechin (3), epicatechin (4), (−)-fisetinidol-(4α,8)-(−)-catechin (5), (4α,8)-(−)-fisetinidol-(−)-epicatechins (6), and (+)-guibourtinidol-(4β,8)-epicatechin (7), were made by our laboratory. These compounds were isolated and identified from ROE by our group and the purity of each standard was higher than 95%.
4; v/v), which were delivered at a flow rate of 0.7 mL min−1 as follows: 0 min, 96% (A); 180 min, 70% (A); 205 min, 96% (A); and 210 min, 0% (A). The UV spectra of every identified peak were recorded at 280 nm. The tested standards, including procyanidin B3 (1), EGC (2), catechin (3), epicatechin (4), (−)-fisetinidol-(4α,8)-(−)-catechin (5), (4α,8)-(−)-fisetinidol-(−)-epicatechins (6), and (+)-guibourtinidol-(4β,8)-epicatechin (7), were made by our laboratory. These compounds were isolated and identified from ROE by our group and the purity of each standard was higher than 95%.
Animals
Adult mice (C57BL/6; 18–20 g weight; 8–12 weeks old) were obtained from the Experimental Animal Center of the Chinese Academy of Military Sciences (license number: SCXK (Beijing) 2014-0013). All the mice were kept in an environment with a temperature of 22–26 °C and a humidity level of 60 ± 5% under a 12 h light/dark cycle with free access to food and water.17 The experiment protocols were approved by the Animal Ethics Committees of Logistics University of Chinese People's Armed Police Forces, Tianjin, China, and performed in accordance with the “Principles of Laboratory Animal Care and Use in Research” (State Council of China, 1988).DSS-induced experimental colitis
Mice were randomly divided into seven groups (6 mice per group) as follows:18 control group (1), where the mice without colitis were treated orally with saline (0.9% NaCl colution); model group (2), where the mice with colitis were given 3% DSS solution as drinking water; treatment groups (3, 4, and 5), where the mice with colitis were given 3% DSS solution as drinking water and treated orally with ROE (125, 250, and 500 mg kg−1, respectively), and the SSZ group (6), where the mice with colitis were given 3% DSS solution as drinking water and treated orally with SSZ (500 mg kg−1). ROE and SSZ were intragastrically given from the 1st day to the 8th day, respectively.Disease activity index (DAI)
The severity of colitis was assessed daily using a DAI based on a scoring system that scored for body weight loss, stool consistency, and rectal bleeding. DAI = (combined score of weight loss, stool consistency, and bleeding)/3.19,20 The DAI was assigned based on the following morphological features using a scale ranging from 0 to 4, as follows: (0) no damage; (1) weight loss of 1% to 5%, slightly loose stool, and hidden blood positive in fecal; (2) weight loss of 5% to 10%, loose stool, and slight blood positive in fecal; (3) weight loss of 10% to 15%, stool trait is ruptured, and gross blood in fecal; and (4) weight loss of more than 15%, watery stool, and dominant bleeding in fecal.Macroscopic assessment
The animals were inspected and weighed daily. After all the animals were sacrificed, blood samples, colonic contents, colon, and spleen tissues were immediately collected. Blood was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min and stored in a refrigerator at −20 °C. The length of the colon was measured by flattening. Then, the colons were removed, opened longitudinally, and washed with phosphate-buffered saline (PBS). Parts of the colon were cut off along the vertical axis, and colonic tissues were immediately homogenized at 4 °C by using a glass homogenizer. The protein concentrations were determined quantitatively using a BCA protein assay kit (Beyotime Biotechnology, USA).
000 g for 10 min and stored in a refrigerator at −20 °C. The length of the colon was measured by flattening. Then, the colons were removed, opened longitudinally, and washed with phosphate-buffered saline (PBS). Parts of the colon were cut off along the vertical axis, and colonic tissues were immediately homogenized at 4 °C by using a glass homogenizer. The protein concentrations were determined quantitatively using a BCA protein assay kit (Beyotime Biotechnology, USA).
Histological and immunohistochemical analysis
The colon was divided into different parts. The same part of each animal's colon was selected and fixed in 10% buffered formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E). Colonic tissue sections were analyzed under a microscope and photographed for preservation.Specific antibodies against Nrf2 and NF-κB were used for immunohistochemical analysis. In brief, 4 μm thick sections were incubated in 10% normal serum containing 1% bovine serum albumin in Tris-buffered saline for 2 h at 25 °C, and then incubated with 1% H2O2 for 15 min. They were incubated with primary antibodies against Nrf2 and NF-κB (dilution, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) overnight at 4 °C. For detection, a biotin-labeled rabbit anti-mouse secondary antibody (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) was added, followed by avidin/biotin-peroxidase detection solution (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Colon specimens were counterstained with hematoxylin, dehydrated with gradient ethanol, sealed with neutral gum, and microscopically examined. The integrated optical densities of Nrf2, and NF-κB in each sample were measured by Iimge-proplus software and expressed as a mean value.
100) overnight at 4 °C. For detection, a biotin-labeled rabbit anti-mouse secondary antibody (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) was added, followed by avidin/biotin-peroxidase detection solution (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Colon specimens were counterstained with hematoxylin, dehydrated with gradient ethanol, sealed with neutral gum, and microscopically examined. The integrated optical densities of Nrf2, and NF-κB in each sample were measured by Iimge-proplus software and expressed as a mean value.
Cell culture
RAW264.7 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, United States), and maintained in DMEM supplemented with 10% FBS, in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The experiment was divided into the following groups: control group (culture medium only), LPS stimulation model group (5 μg mL−1 LPS), ROE low dose group (5 μg mL−1 LPS + 100 μg mL−1), ROE middle dose group (5 μg mL−1 LPS + 200 μg mL−1), and ROE high dose group (5 μg mL−1 LPS + 400 μg mL−1).Cell viability assay
Cell viability was measured by MTT assay.21 Briefly, RAW264.7 cells were seeded at a density of 1 × 104 cells per well in 96-well plates. Then, the cells were treated with different concentrations of ROE (1, 10, 100, 200, 400, 600, 800, 1000 μg mL−1) in the presence of 5 μg mL−1 LPS for 24 h in 96-well plates. Next, 10 μL of MTT solution (5 mg mL−1) was added to the culture medium, and the cells were incubated for 2 h. Finally, cell culture supernatants were removed, then DMSO was added to each well to dissolve any formazan crystals that had formed. The absorbance was measured at 490 nm using a Spectra microplate reader (Infinite F50, Switzerland Tecan). Each group of samples was subjected to three parallel experiments.Detection of the levels of NOS, MPO, SOD, and MDA
The serum and colon homogenate supernatants of the animals were collected to detect the levels of NOS, MPO, SOD, and MDA. The experimental procedure was carried out according to the instructions of the commercial kits. The absorbance was measured using a microplate reader.RAW264.7 cells (1 × 104 cells per well) were treated with increasing concentrations of ROE in the presence of 5 μg mL−1 LPS for 24 h in 96-well plates. The supernatant was then removed, and the NOS content of each well was determined according to the manufacturer's instructions.
Detection of the levels of inflammatory cytokines
The levels of TNF-α, IL-6, and IL-1β in the serum and colon tissues of the mice were detected by ELISA according to the manufacturer's instructions. Briefly, RAW264.7 cells were seeded in 96-well plates with a density of 1 × 104 cells per well for 24 h. The cells were pretreated with ROE for 1 h, and then stimulated with LPS (5 μg mL−1) for 24 h. The contents of TNF-α, IL-6, and IL-1β in the cell supernatant were determined using commercial ELISA kits.Western blotting analysis
Western blot analysis of the mice was carried out by the method in ref. 22. The colon tissues of the mice were collected and homogenized with ice-cold RIPA lysis buffer (Com Win Biotech Co. Ltd., Beijing, China) containing PMSF protease inhibitors (Solarbio Science & Technology Co. Ltd., Beijing, China) to extract the total protein. The homogenate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g at 4 °C for 15 min. The amount of total extracted protein was determined by BCA TM protein assay kit. An equal amount of protein was separated by 10% SDS-PAGE and was then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skim milk for 1 h at room temperature to prevent non-specific binding and incubated with primary antibody at 4 °C overnight. The following primary antibodies and dilutions were used: Nrf2, Keap1, HO-1, phospho-NF-κB-p65, phospho-IKKα/β (1
000 g at 4 °C for 15 min. The amount of total extracted protein was determined by BCA TM protein assay kit. An equal amount of protein was separated by 10% SDS-PAGE and was then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skim milk for 1 h at room temperature to prevent non-specific binding and incubated with primary antibody at 4 °C overnight. The following primary antibodies and dilutions were used: Nrf2, Keap1, HO-1, phospho-NF-κB-p65, phospho-IKKα/β (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), and β-actin (1
1000 dilution), and β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution). On the following day, the membrane with the primary antibodies was washed in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) 3 times, and then incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies (1
000 dilution). On the following day, the membrane with the primary antibodies was washed in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) 3 times, and then incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution; anti-mouse, Cat. no. sc-2005; anti-rabbit, Cat. no. sc-2030, Santa Cruz Biotechnology, Inc.). The immunoreactive protein bands were visualized using an enhanced chemiluminescence (ECL) reagent kit (ECL; Millipore Corporation, Billerica, MA 01821 USA).
000 dilution; anti-mouse, Cat. no. sc-2005; anti-rabbit, Cat. no. sc-2030, Santa Cruz Biotechnology, Inc.). The immunoreactive protein bands were visualized using an enhanced chemiluminescence (ECL) reagent kit (ECL; Millipore Corporation, Billerica, MA 01821 USA).
RAW264.7 cells (4 × 105 cells per mL) were pretreated with or without ROE (100, 200, 400 μg mL−1) for 1 h in 6-well plates. Then, the cells with or without LPS (5 μg mL−1) were incubated for 24 h. RAW 264.7 cells were harvested on ice and washed in ice-PBS after treatment. The total proteins of each group were respectively extracted with RIPA lysis buffer containing PMSF protease inhibitors and quantitated by BCA protein assay kit. According to the manufacturer's instructions, the extraction of nuclear protein with the appropriate kit (Key GEN BioTECH, Jiangsu, China) was used to obtain the nuclear proteins. The expression of Nrf2, Keap1, HO-1, p-NF-κB-p65, and p-IKKα/β proteins were detected as described above.
Statistical analysis
Date are presented as the mean ± SD or the mean ± S.E.M. Statistical analysis was performed with one way analysis of variance (ANOVA) followed by the Bonferroni post hoc test using GraphPad Prism software (GraphPad Prism version 6.07, San Diego, CA, USA). Results were considered as statistically significant when p < 0.05.Results
HPLC profile of ROE
The HPLC fingerprint of ROE revealed the presence of ten peaks with retention times ranging from 29.5 to 122 min (Fig. 1). Based on the UV-visible spectral data, the retention times, and by comparing with the standards, ROE showed the presence of procyanidin B3 (1), EGC (2), catechin (3), epicatechin (4), (−)-fisetinidol-(4α,8)-(−)-catechin (5), (4α,8)-(−)-fisetinidol-(−)-epicatechins (6), and (+)-guibourtinidol-(4β,8)-epicatechin (7).Anti-inflammatory effects of ROE in DSS-induced colitis
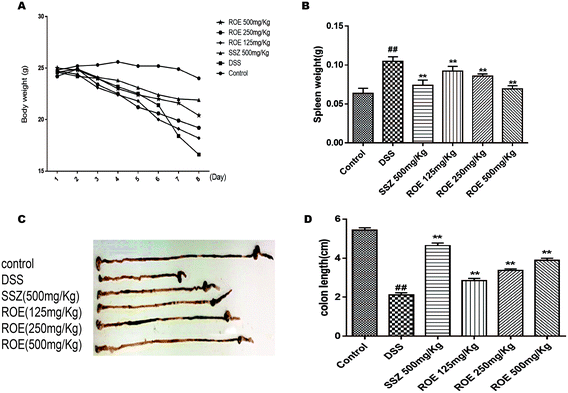
DSS-induced UC in mice is a well-established preclinical model that exhibits many phenotypic features according to human ulcerative colitis.23 In order to examine whether ROE could improve the clinical symptoms of DSS-induced colitis in mice, the clinical signs, including weight changes, colon length, and DAI score, were considered. As shown in Table 1, ROE and SSZ produced a significant improvement in DAI score compared with that in the model group. The DSS-induced colitis mice exhibited dramatic body weight loss and spleen weight loss, which was reversed by ROE markedly (Fig. 2A and B). As shown in Fig. 2C and D, in the DSS-treated mice, colon shortening, a marker of colitis, was significantly ameliorated by ROE. Therefore, these results showed that the DSS-induced colon damage could be restored by ROE in a dose-dependent manner.| Group | Day 1 | Day 3 | Day 5 | Day 8 |
|---|---|---|---|---|
| a p < 0.01 vs. control group.b p < 0.01 vs. model group. | ||||
| Control | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 3% DSS | 0.0 ± 0.00 | 1.47 ± 0.01a | 3.16 ± 0.03a | 3.93 ± 0.03a |
| SSZ (500 mg kg−1) | 0.0 ± 0.00 | 0.82 ± 0.02b | 1.21 ± 0.14b | 1.28 ± 0.00b |
| ROE (125 mg kg−1) | 0.0 ± 0.00 | 0.92 ± 0.13b | 1.78 ± 0.01b | 2.88 ± 0.02b |
| ROE (250 mg kg−1) | 0.0 ± 0.00 | 0.87 ± 0.01b | 1.36 ± 0.02b | 2.15 ± 0.18b |
| ROE (500 mg kg−1) | 0.0 ± 0.00 | 0.86 ± 0.02b | 1.08 ± 0.12b | 1.93 ± 0.01b |
ROE diminished colonic histopathological changes
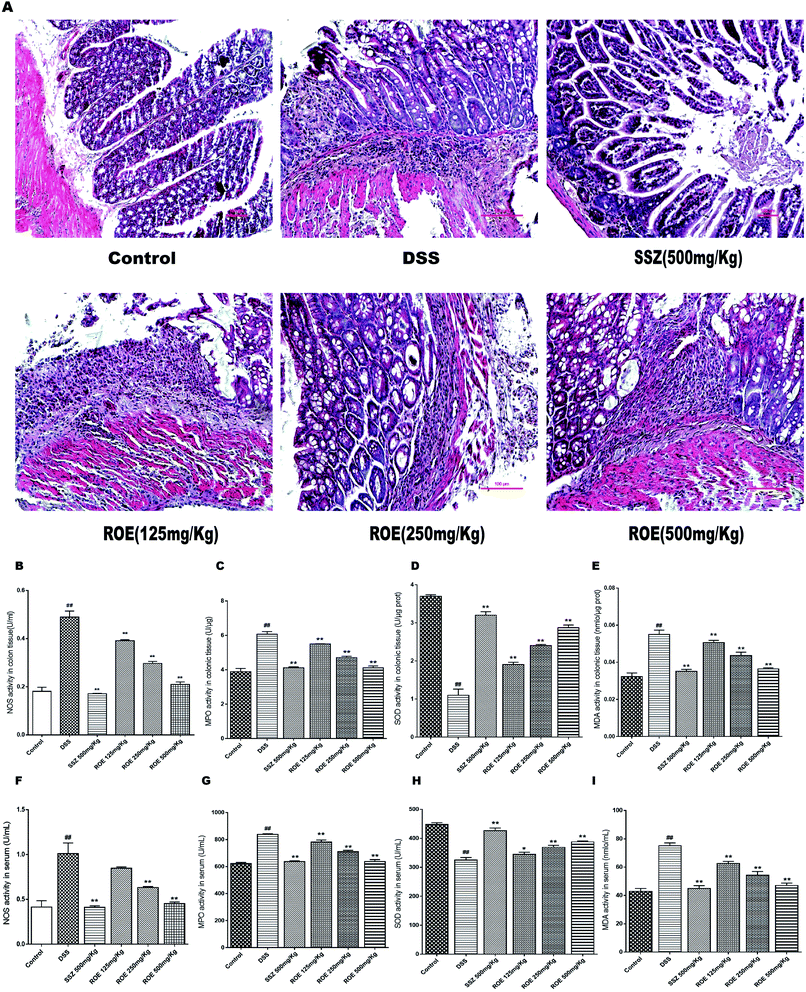
We then investigated whether ROE could alter histopathological damage in the colons of mice with DSS-induced colitis. The results showed that the colon tissues from the model group revealed typical characteristics of an abnormal structure, including the loss of epithelial and goblet cells, crypt lesions, and prominent transmural inflammatory cells infiltration in the intestine mucosa and submucosa. ROE and SSZ could ameliorate the histopathological changes caused by DSS (Fig. 3A).ROE inhibited oxidative stress and increased antioxidant products in DSS-induced colitis
The levels of NOS, MDA, MPO, and SOD in the serum and colon tissues of mice were further measured (Fig. 3B–I). The results showed that the secretion of NOS, MDA, and MPO were obviously increased after DSS challenge, which was remarkably suppressed by ROE. Compared with the model group, the SOD content increased with the dose increase in the administration group of the ROE.ROE decreased the production of inflammatory cytokines in DSS-induced colitis
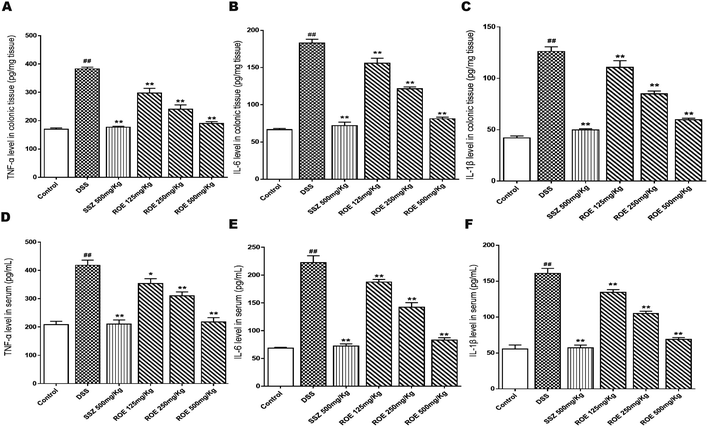
To gain insights into the inflammatory milieu associated with DSS-induced colitis, we measured the levels of inflammatory cytokines using ELISA, and observed severe inflammatory responses in the DSS-induced mice. ROE could inhibit significantly the levels of TNF-α, IL-6, and IL-1β in the serum and colon tissues of UC mice (Fig. 4).ROE suppressed NF-κB and activated the Nrf2 signaling pathway in DSS-induced colitis
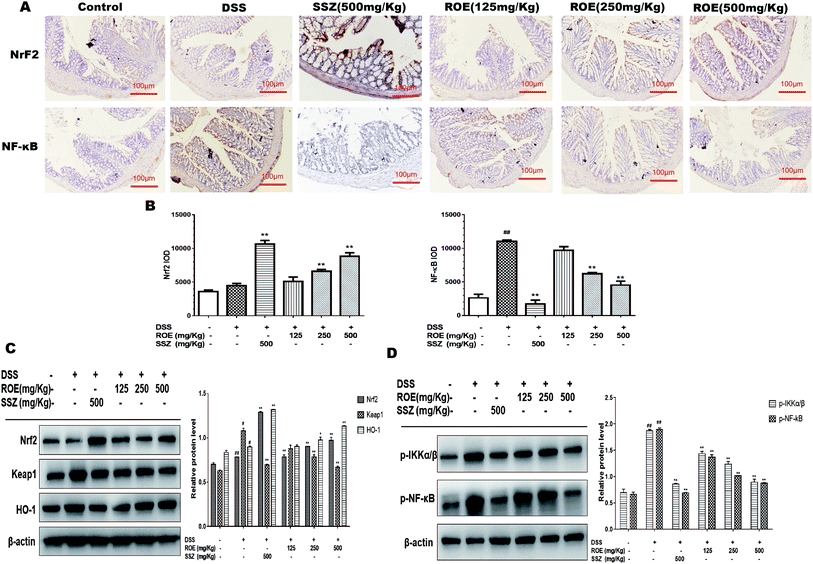
To further elucidate the detailed mechanisms of ROE, we investigated the expression of Nrf2 and p-NF-κB-p65 in the colon tissues of DSS-induced colitis, both of which have been well documented to be involved in inflammation. As shown in Fig. 5A and B, the positive expression of NF-κB-p65 was much higher in the colon tissues of DSS-induced mice compared with that of the control mice. However, 500 mg kg−1 ROE dramatically reduced the number of nuclear NF-κB-p65 positive cells. In addition, ROE administration increased the expression of Nrf2 compared with that of the model group. Moreover, the expression of Nrf2 and HO-1 protein was remarkably increased, and the expression of p-NF-κB-p65, p-IKKα/β, and Keap1 was dramatically inhibited by ROE treatment in a dose-dependent manner (Fig. 5C and D). These results indicated that regulation of the NF-κB and Nrf2 pathways may underlie the beneficial effects of ROE in mice with DSS-induced colitis.ROE reduced the production of pro-inflammatory cytokines in RAW264.7 cells
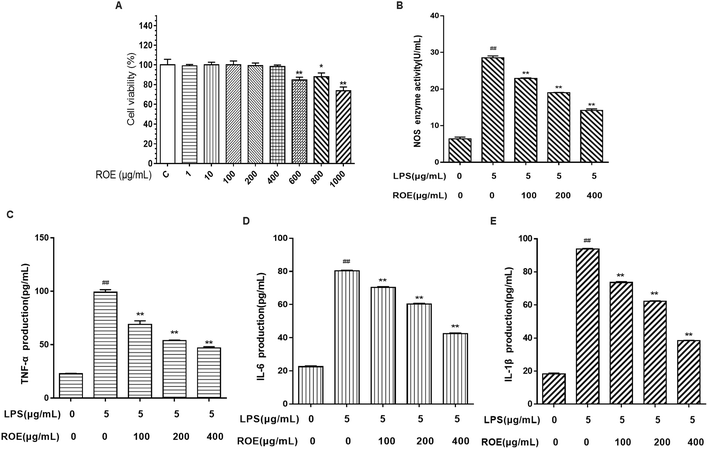
As shown in Fig. 6A, the results indicated that ROE (1, 10, 100, 200, 400 μg mL−1) displayed no significant toxicity in RAW264.7 cells compared with the control (C). Our results demonstrated that ROE treatment markedly lessened the activity of NOS in RAW264.7 cells stimulated with LPS dose-dependently.To evaluate whether ROE affected the levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, RAW264.7 cells were treated with ROE in the presence of LPS. As shown in Fig. 6C–E, pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were induced by LPS in RAW264.7 cells. ROE treatment significantly suppressed the production of these pro-inflammatory cytokines in a dose-dependent manner. These results demonstrated that ROE could exert anti-inflammatory effects by inhibiting the production of several pro-inflammatory mediators.
ROE exerted anti-inflammatory effects via the Nrf2 and NF-κB pathways in vitro
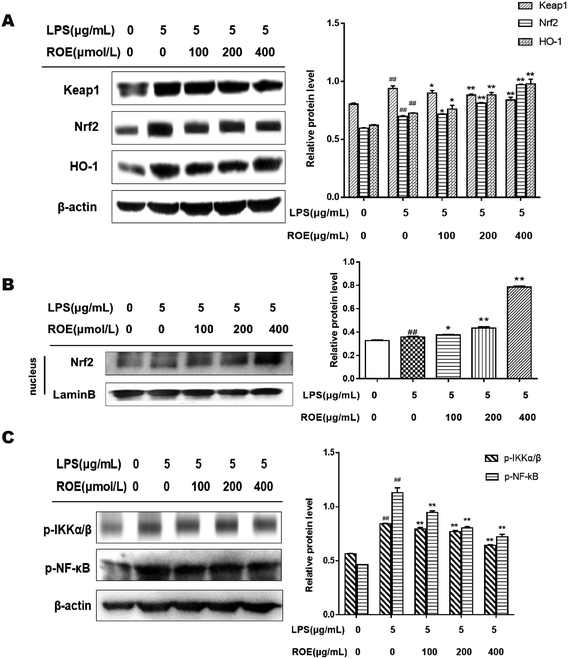
To understand the mechanisms of the inhibition of inflammation in LPS-stimulated RAW264.7 cells by ROE, we evaluated the effects of ROE on the activation of the Nrf2 and NF-κB pathways. As shown in Fig. 7A and B, ROE could increase the expression of Nrf2 and HO-1 protein dose-dependently. At the same time, there was an intense activation of NF-κB with the upregulation of p-NF-κB-p65, p-IKKα/β, and Keap1 after treatment with LPS in RAW264.7 cells as compared with the control, which were significantly inhibited by ROE (Fig. 7C). These results suggested that ROE might exert its inflammation suppressive effect through the activation of the Nrf2 and inhibition of the NF-κB signaling pathways.Discussion
UC is a chronic and nonspecific inflammatory disorder primarily involving the mucosa and submucosa of the colon. Multiple factors, such as the overproduction of pro-inflammatory mediators and neutrophil infiltration, have been implicated in the pathogenesis of colitis.24 UC affects 8–12 individuals per hundred thousand individuals. From the updated studies, the current drugs commonly used to treat UC include amino salicylates, immunomodulators, steroids, and some biologics. However, all of them can easily lead to a significant loss of patient compliance, and potential toxic or side effects. Consequently, it is imperative to develop novel therapeutics with high efficacy and safety.25,26Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils has the effects of stopping diarrhea and intestinal tract, and has been used for treating intestinal diseases, such as diarrhea and bacillary dysentery. Our previous studies have shown that ROE could inhibit the contraction of isolated intestinal smooth muscle induced by acetylcholine stimulation. However, the mechanisms underlying the protective effects of ROE on UC are still under investigation. In the present study, we investigated the anti-inflammatory effects and the underlying mechanisms of ROE, both in DSS-induced UC mice and in LPS-stimulated RAW264.7 cells.
The DSS-induced colitis model has the same hallmark with human inflammatory bowel disease to some extent.27 SSZ is believed to have unique effects in the treatment of ulcerative colitis. Here, we first evaluated the protective efficacy of ROE on UC using SSZ as a reference. Our results demonstrated that ROE could markedly improve colonic damage and the inflammatory symptoms in DSS-induced UC mice, including colon length shortening, weight loss, and colon tissue damage, and reduce DAI compared with the model group. The results of H&E staining showed that ROE decreased significantly the levels of inflammatory cells infiltration, mucosal edema, and alleviated the adhesion to the colon wall in UC mice.
Oxidative stress is a risk factor for the development of chronic inflammatory diseases, such as UC, and is the main driving inflammation toward DNA damage, which in turn leads to carcinogenesis.28 MDA is a product of oxygen free radicals triggering lipid peroxidation of unsaturated fatty acids on cell membranes,29 and SOD is an important antioxidant enzyme in living organisms.30 Furthermore, the activity of NOS can reflect the content of NO, while the content of NO is also a reference index of inflammation for UC.31 NO could lead to tissue damage in the middle and distal colon through the infiltration of neutrophils, and MPO is a well-established biomarker of neutrophil infiltration for evaluating inflammation in humans and animal models of UC.32 In this study, our data showed that the activity of SOD in the model group was much lower, meanwhile the contents of MDA, MPO, and NOS were significantly higher than those of the control group, indicating the oxidative stress in the UC mouse model. After treatment with ROE, the SOD activity was increased, and the levels of MDA, MPO, and NOS were decreased. In addition, the NOS activity in the LPS-induced RAW 264.7 was also decreased by ROE.
The inflammatory response is an important indicator of the inflammatory state of the body. The determination of IL-6, IL-1β, and TNF-α plays an important guiding role in evaluating the inflammatory state of the body. The overexpression of TNF-α, IL-6, and IL-1β is closely linked to intestinal inflammation and mucosal inflammation of UC, which may cause damage to the intestinal mucosa and tract.33 The high level of pro-inflammatory cytokine production is a major character of DSS-induced colitis.34 TNF-α is involved in systemic inflammation, and the overexpression of TNF-α could lead to intestinal mucosal impairment.35,36 IL-6 can cause the colon damage leading to inflammation, and IL-1β can cause auto-immune processes that damage the colon tissue.37 Our data showed that the levels of TNF-α, IL-6, and IL-1β were significantly inhibited by ROE, both in the DSS-induced UC mice and in the LPS-induced RAW264.7 cells. These results demonstrated that ROE could alleviate UC via the regulation of oxidative stress and the inhibition of inflammation.
To gain further insights into the mechanisms through which ROE exerts its anti-inflammatory effect in DSS-induced colitis, we assessed the key proteins of the NF-κB pathway. NF-κB is generally considered to exist in the cytoplasm as a heterodimer complex of p65/p50 subunits combined with an inhibitory protein, IκB. Inflammatory stimuli induce the phosphorylation and consequent degradation of IκB by IκB kinase (IKK), which allows NF-κB translocation into the cell nucleus, binding to target DNA elements, and activating the transcription of genes encoding proteins involved in inflammation responses.38 In the present study, the expressions of p65 NF-κB and IKKα/β were significantly increased in DSS-induced UC mice, while administration with ROE downregulated the levels of p65 NF-κB and IKKα/β. Thus, we assumed that ROE could prevent the degradation of IKKα/β, thereby inhibiting nuclear localization of NF-κB, which resulted in the amelioration of DSS-induced colitis.
The question then arises as to how ROE exerts its anti-inflammatory effect on DSS-induced colitis through NF-κB mediated reactions. It is well documented that ROS activates NF-κB, leading to the generation of pro-inflammatory cytokines and inducible enzymes. Meanwhile, the pro-inflammatory cytokines cause oxidative stress by promoting the release of ROS. Thus, inflammation and oxidative stress are also involved in the spiraling vicious cycle that contributes to the severity of the UC.39 Nrf2 is a key transcription factor that regulates the expression of cytoprotective genes in response to oxidative stress. Under conditions of homeostatic cell growth, the cytoplasmic protein Keap1 interacts with Nrf2, and represses its function. However, under oxidative stress conditions, Nrf2 is released from Keap1 and is translocated into the nucleus, and then binds to the antioxidant responsive element (ARE), resulting in the activation of various antioxidant enzymes, such as SOD and HO-1.40 In the present study, we observed a marked decrease of NOS and marked elevation of SOD in the colon tissues of UC mice treated with ROE, suggesting that ROE had an antioxidant effect. Western blot analysis results showed an increased expression of Nrf2 and HO-1, and decreased expression of Keap1 (Nrf2 repressor) in the ROE-treated mice. These above results indicated that ROE inhibited NF-κB and promoted Nrf2 activation to decrease the DSS-induced inflammation injury. This was the first time that it has been found that ROE exerts a protective effect via regulating the NF-κB/Nrf2 signaling pathway in DSS-induced UC mice. However, the detailed mechanisms still need further study.
Conclusion
In conclusion, our data have demonstrated that treatment with ROE is effective for the prevention of DSS-induced colitis in mice through the suppression of inflammation and oxidative stress, which are mechanisms that might be involved in the inhibition of NF-κB and the activation of the Nrf2 pathway, both in a DSS-induced-UC mouse model and in LPS-stimulated RAW264.7 cells (Fig. 8). Therefore, ROE might be a novel and attractive chemoprevention agent for UC and inflammation-related diseases.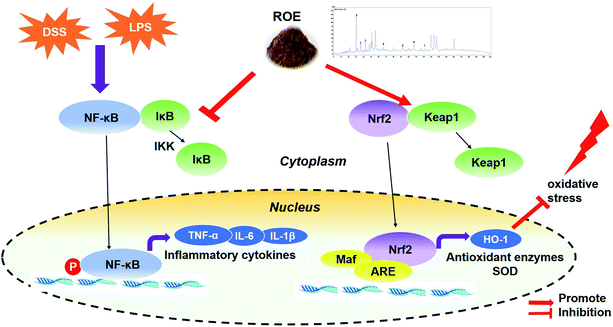 | ||
| Fig. 8 A proposed model for the mechanism of ROE against DSS-induced UC and in LPS-stimulated RAW264.7 cells. | ||
Abbreviation
| ROE | The extracts from the roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils |
| LPS | Lipopolysaccharide |
| ROS | Reactive oxygen species |
| Nrf2 | Nuclear factor-E2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| HO-1 | Heme oxygenase-1 |
| NQO1 | Benzoquinone oxidoreductase |
| SOD | Superoxide dismutase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| DSS | Dextran sulfate sodium |
| NOS | Nitric oxide synthase |
Conflicts of interest
We have no conflicts of interest in this research.Acknowledgements
We thank the National Natural Science Foundation of China (81673693) and the National Key R&D Program of China (2017YFD0201402) for financial support. The work was also supported by the Scientific Research Innovation Team Foundation of the Logistics University of CPAPF (WHTD2018).References
- H. Zhou, H. J. Zhang, L. Guan, Y. N. Zhang, Y. Li and M. J. Sun, Mol. Med. Rep., 2018, 18, 332–343 Search PubMed.
- I. V. Byelinska, H. M. Kuznietsova, N. V. Dziubenko, O. V. Lynchak, T. V. Rybalchenko, Y. I. Prylutskyy, O. A. Kyzyma, O. Ivankov, V. K. Rybalchenko and U. Ritter, Mater. Sci. Eng., C, 2018, 93, 505–517 CrossRef CAS PubMed.
- C. W. Yuan, X. L. Sun, L. C. Qiao, H. X. Xu, P. Zhu, H. J. Chen and B. L. Yang, World J. Gastroenterol., 2019, 25, 6813–6822 CrossRef PubMed.
- H. Y. Jeong, Y. S. Choi, J. K. Lee, B. J. Lee, W. K. Kim and H. Kang, Nutrients, 2017, 9, 1–12 Search PubMed.
- L. H. Pan, C. H. Sun, J. Yang, J. C. Yao, B. B. Li, Y. J. Tan, G. M. Zhang and Y. Sun, Chin. Herb. Med., 2018, 10(1), 40–45, DOI:10.1016/j.chmed.2017.12.005.
- Q. L. Zhang, J. Zhang, P. F. Xia, X. J. Peng, H. L. Li, H. Jin, J. Yang and L. Zhao, Chin. Herb. Med., 2019, 11, 108–112, DOI:10.1016/j.chmed.2018.10.004.
- S. J. Qiu, P. Li, H. F. Zhao and X. F. Li, Int. Immunopharmacol., 2020, 78, 1–7 Search PubMed.
- M. Sabzevary-Ghahfarokhi, M. Shohan, H. Shirzad, G. Rahimian, A. Soltani, M. Ghatreh-Samani, F. Deris, N. Bagheri, M. Shafigh and K. Tahmasbi, Pathol., Res. Pract., 2018, 214, 1149–1155 CrossRef CAS PubMed.
- N. Eissa, H. Hussein, L. Kermarrec, O. Elgazzar, M. H. Metz-Boutigue, C. N. Bernstein and J. E. Ghia, Biochem. Pharmacol., 2017, 145, 102–113 CrossRef CAS.
- J. K. Triantafillidis, E. Merikas and F. Georgopoulos, Drug Des., Dev. Ther., 2011, 5, 185–210 CrossRef CAS.
- G. Rogler, Best Pract. Res., Clin. Gastroenterol., 2010, 24, 157–165 CrossRef CAS PubMed.
- W. Feng, H. Ao, S. J. Yue and C. Peng, Sci. Rep., 2018, 8, 1–12 CrossRef CAS PubMed.
- H. Y. Shi, C. L. Wang, Y. M. Zhao, T. K. Xue, D. L. Liu, X. Y. Wei, S. Y. Qi and L. Chao, Drug Eval. Res., 2017, 40, 1581–1586, DOI:10.7501/j.issn.1674-6376.2017.11.010.
- S. Xie, Z. P. Tong, R. Tan and X. Z. Liu, China J. Chin. Mater. Med., 2014, 39, 2912–2914, DOI:10.4268/cjcmm20141522.
- J. J. Li, X. Q. Hu, X. F Zhang, J. J. Liu and L. S. Cao, China J. Chin. Mater. Med., 2014, 39, 4246–4251, DOI:10.4268/cjcmm20142131.
- Y. An, G. M. Song, D. L. Liu, T. Y. Mao, Y. M. Zhao and W. L. Xie, Pak. J. Pharm. Sci., 2018, 31, 835–840 CAS.
- A. M. Abdelmegid, F. K. Abdo, F. E. Ahmed and A. A. A. Kattaia, Sci. Rep., 2019, 9, 1–16 CrossRef CAS PubMed.
- S. Y. Zong, T. Q. Pu, B. L. Xu, T. Zhang and B. Wang, Int. Immunopharmacol., 2017, 42, 32–38 CrossRef CAS.
- H. Li, C. Fan, C. Feng, Y. Wu, H. Lu, P. He, X. Yang, F. Zhu, Q. Qi, Y. Gao and J. Zuo, Br. J. Pharmacol., 2019, 36, 1429–1437 Search PubMed.
- J. Wang, C. Zhang, C. Guo and X. Li, Int. J. Mol. Sci., 2019, 20, 1–12 Search PubMed.
- C. Zhang, C. Li, X. Jia, K. Wang, Y. Tu, R. Wang, K. Liu, T. Lu and C. He, J. Cancer Mol., 2019, 24, 1–17 CAS.
- J. Song, W. Zhang, J. Wang, H. Yang, X. Zhao, Q. Zhou, H. Wang, L. Li and G. Du, J. Cancer Mol., 2018, 63, 299–310 CAS.
- H. Zhao, N. Cheng, W. Zhou, S. Chen, Q. Wang, H. Gao, X. Xue, L. Wu and W. Cao, Mol. Nutr. Food Res., 2019, 63, 1–34 Search PubMed.
- L. Zong, Z. Gao, W. Xie, J. Tong and Y. Cao, J. Toxicol. Environ. Health, Part A, 2019, 82, 1–6 CrossRef PubMed.
- J. Yao, J. Wang, L. Liu, Y. X. Li, A. Y. Xun, W. S. Zeng, C. H. Jia, X. X. Wei, J. L. Feng, L. Zhao and L. S. Wang, Arch. Med. Res., 2010, 41, 288–294 CrossRef CAS PubMed.
- A. L. Xu, B. M. Liu, D. M. Sun, X. L. Bi, D. K. Cai, Z. H. Chen, X. J. Huang, J. Y. Jiang and P. Ding, Chin. Herb. Med., 2019, 11, 379–386, DOI:10.1016/j.chmed.2019.08.002.
- S. Y. Cao, S. J. Ye, W. W. Wang, B. Wang, T. Zhang and Y. Q. Pu, Chin. J. Nat. Med., 2019, 17, 81–102 Search PubMed.
- J. Shen, J. Cheng, S. Zhu, J. Zhao, Q. Ye, Y. Xu, H. Dong and X. Zheng, Int. Immunopharmacol., 2019, 73, 193–200 CrossRef CAS PubMed.
- L. Guan, X. Geng, C. Stone, E. E. P. Cosky, Y. Ji, H. Du, K. Zhang, Q. Sun and Y. Ding, Environ. Toxicol., 2019, 34, 530–538 CrossRef CAS PubMed.
- J. M. Lü, J. Jiang, M. S. Jamaluddin, Z. Liang, Q. Yao and C. Chen, Int. J. Mol. Sci., 2019, 20, 1–17 Search PubMed.
- N. O. Al-Harbi, F. Imam, M. M. Al-Harbi, O. A. Al-Shabanah, M. R. Alotaibi, H. M. As Sobeai, M. Afzal, I. Kazmi and A. C. Al Rikabi, Int. Immunopharmacol., 2019, 27, 817–827 CAS.
- N. M. Aborehab, M. H. El Bishbishy, A. Refaiy and N. E. Waly, BMC Complementary Altern. Med., 2017, 17, 1–9 CrossRef PubMed.
- Y. Guo, X. Wu, Q. Wu, Y. Lu, J. Shi and X. Chen X, Toxicol. Appl. Pharmacol., 2018, 344, 35–45 CrossRef CAS PubMed.
- A. C. Moldoveanu, M. Diculescu and C. F. Braticevici, Rom. J. Intern. Med., 2015, 53, 118–127 Search PubMed.
- I. Floris, K. Appel, T. Rose and B. Lejeune, J. Inflammation Res., 2018, 11, 397–405 CrossRef CAS PubMed.
- B. Cao, R. B. Sun, G. Yan, G. Y. Yang, J. Y. Aa and J. Li, Chin. Herb. Med., 2019, 11, 292–298, DOI:10.1016/j.chmed.2019.05.004.
- J. Zhang and M. L. Chu, J. Inflammation, 2019, 11, 397–405 Search PubMed.
- X. Huo, L. Zhang, L. Gao, Y. Guo, L. Zhang, L. Li, J. Si and L. Cao, Biol. Pharm. Bull., 2015, 38, 1328–1336 CrossRef CAS PubMed.
- J. E. Toblli and G. Cao, Drug Des., Dev. Ther., 2015, 9, 2585–2587 CrossRef CAS PubMed.
- B. Harder, T. Jiang, T. Wu, S. Tao, M. Rojo de la Vega, W. Tian, E. Chapman and D. D. Zhang, Biochem. Soc. Trans., 2015, 43, 680–686 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10747a |
| ‡ These two authors contributed equally to this work as the first author. |
| This journal is © The Royal Society of Chemistry 2020 |