Open Access Article
Open Access ArticleA mild and metal-free synthesis of 2- and 1-alkyl/aryl/dialkyl-aminoquinolines and isoquinolines†
Yerramsetti Nanaji a,
Seema Kirarb,
Sandip V. Pawar
a,
Seema Kirarb,
Sandip V. Pawar *c and
Ashok Kumar Yadav
*c and
Ashok Kumar Yadav *c
*c
aTexas Tech University Health Sciences Center, Ophthalmology Department Lubbock General, 3601 4th Street, Lubbock, TX 79430, USA
bDepartment of Biotechnology, National Institute of Pharmaceutical Education and Research (NIPER), Sector-67, S. A. S. Nagar-160062, Punjab, India
cUniversity Institute of Pharmaceutical Sciences, Panjab University, Chandigarh-160014, India. E-mail: pawars@pu.ac.in; ashoky@pu.ac.in
First published on 20th February 2020
Abstract
A simple synthetic strategy has been developed for the synthesis of 2- and 1-alkyl/aryl/dialkylaminoquinolines and isoquinolines from the easily available quinoline and isoquinoline-N-oxides, different amines, triflic anhydride as activating agent and acetonitrile as solvent in a one-pot reaction under metal-free conditions at 0 °C to room temperature.
Introduction
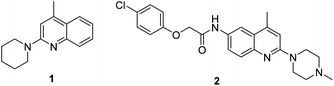
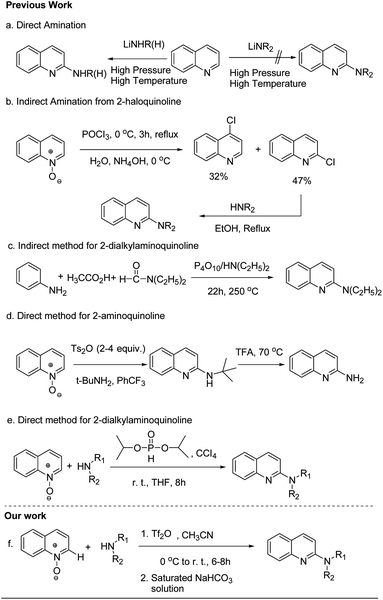
The basic motifs of 2-aminoquinolines and 1-aminoisoquinolines are present in a number of alkaloids1 that have a broad range of biological activities, including antimicrobial activity,2 anti-Alzheimer disease,3 anti-HIV,4 antihelmintic,5 antidepressant,6 and antihypertensive7 activities. This type of skeleton-containing molecule is an interesting target as potent leads for the medicinal chemist. Some representative examples are given below in Fig. 1. Compound 1 selectively modulates native TRPC4/C5 ion channels and is a potent antagonist. This compound has a broad scope in physiological and pathophysiological studies,1b whereas compound 2, as an antagonist of MCH-1R, is used for the treatment of obesity.2cThere are several reports in the literature for the synthesis of 2-aminoquinoline and 1-aminoisoquinoline derivatives.8 The Chichibabin reaction is one of them, in which amino or alkylamino groups can be incorporated directly into the quinoline and isoquinoline nucleus by the reaction of quinoline and isoquinoline with alkali amide or alkylamide. The Chichibabin reaction does, however, have some drawbacks, such as low yields, functional group intolerance and poor regioselectivity due to strong basic conditions, high temperatures and longer reaction times (Scheme 1a).9 Earlier, it was noted that 2-(dialkylamino)quinolines/(1-dialkylamino)isoquinolines cannot be prepared by other variants of the Chichibabin reaction. This shows that we cannot introduce dialkylamino groups into the quinoline/isoquinoline nucleus by the use of alkali dialkylamides.10 A literature survey shows that derivatization of the 2-unsubstituted quinoline moiety to the corresponding 2-dialkylaminoquinoline was obtained via indirect synthetic methods. The other important approach is amination of 2-haloquinolines with alkyl/dialkylamines.11 However, to use this approach, first, a halogen atom should be incorporated at the 2-position of quinoline and its derivatives, which is achieved by chlorination of quinoline-N-oxides with 2- and 4-regioselectivity and poor yields (Scheme 1b).11 Londregan reported the amination method for the synthesis of 2-aminopyridines, and when 2-cyclohexylamino-quinoline was made utilising this method, poor yield was observed. They used the phosphonium salt PyBroP as the activating agent in this reaction, which is expensive.12 Pedersen also described the synthesis of 2-(dialkylamino)quinolines by the reaction of acetanilides and N,N-dialkylformamides in the presence of phosphorus pentoxide and a dialkylamine at 250 °C.13a This method has drawbacks of high temperature, prolonged reaction time, and poor yield (Scheme 1c). Further, Yin and Xiang reported a two-step synthetic route for the synthesis of 2-aminoquinolines in which an expensive solvent, PhCF3, was used, and excess (5–9 equiv.) of t-BuNH2 was needed to react with quinoline-N-oxide in the first step to form N-(t-butyl)-substituted 2-aminoquinolines (Scheme 1d).13b Zhuo developed a methodology for the preparation of 2-dialkylaminoquinolines from quinoline-N-oxides, diisopropyl H-phosphonate, tertiary amines and carbon tetrachloride under metal-free reaction conditions at room temperature (Scheme 1e)14 and the limitation of this reaction is the use of symmetrical tertiary amine. In 2017, Karchava reported a simple, one-pot preparation of N-(2-pyridyl)-N-ethyl-piperazines15 from pyridine-N-oxide and 1,4-diazabicyclo[2.2.2]octane (DABCO), which generates N-(2-pyridyl)-DABCO salt and further ring opening yields the product by nucleophilic attack. Hence, the development of a simple and handy method for the synthesis of 2-(alkyl/aryl/dialkyl-amino)quinolines and 1-(alkyl/aryl/dialkylamino)quinolines from easily available starting materials without the use of metal is still needed.
Results and discussion
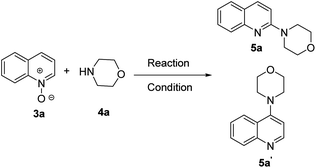
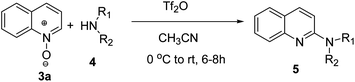
Here, we report a synthetic method by which a series of 2- and 1-alkyl/aryl/dialkylaminoquinolines and isoquinolines are easily prepared by reaction of quinoline and isoquinoline-N-oxides with different alkyl/aryl/dialkylamines at 0 °C to room temperature in the presence of triflic anhydride as activator and acetonitrile as solvent in a one-pot reaction (Scheme 1f).We began our study to optimize reaction conditions for the synthesis of 2-morpholinoquinoline, 5a, between reaction of quinoline-N-oxide, 3a, and morpholine, 4a, in the presence of triflic anhydride as activator under different reaction conditions, as shown in Scheme 2 and Table 1 (entries 1–9). It was found that 2-morpholinoquinoline 5a was obtained in good yield (82%) when the N-oxide of quinoline 3a (1.0 equiv.) was reacted with morpholine 4a (1.2 equiv.) and triflic anhydride (Tf2O) (1.5 equiv.) in acetonitrile as solvent at 0 °C to room temperature for 8 h (Table 1, entry 9). There is also the possibility of formation of the isomeric 4-morpholinoquinoline 5a′. Compound 5a′ was never observed.
| Entry | Reaction condition | % yield of product 5a |
|---|---|---|
| a 5a was observed in TLC and could not be isolated. | ||
| 1 | CH2Cl2, Tf2O, 0 °C to rt, 12 h | No reaction |
| 2 | Et2O, Tf2O, 0 °C to rt, 12 h | No reaction |
| 3 | Toluene, Tf2O, 0 °C to rt, 12 h | aTrace product |
| 4 | CH3CN, Tf2O (2 equiv.), 0 °C to rt, 8 h | 80% |
| 5 | DMSO, Tf2O, 0 °C to rt, 12 h | aTrace product |
| 6 | THF, Tf2O, 0 °C to rt, 12 h | aTrace product |
| 7 | THF, t-BuOK, 0 °C to rt, 12 h | aTrace product |
| 8 | THF, NaH, 0 °C to rt, 12 h | aTrace product |
| 9 | CH3CN, Tf2O (1.5 equiv.), 0 °C to rt, 8 h | 82% |
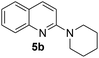
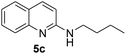
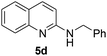
The above optimised reaction conditions were employed for the synthesis of other 2-alkyl/aryl/dialkylamino-substituted quinolines (5b–l) as shown in Scheme 3 and Table 2.
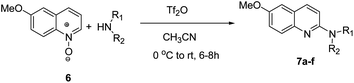
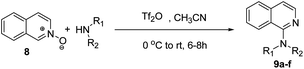
Further, the optimized methodology was extended for the synthesis of 2-alkyl/aryl/dialkyl-aminosubstituted-6-methoxy-quinolines 7a–f from the reaction of 5-methoxyquinoline-N-oxide (6) with different amines (Scheme 4 and Table 3). Next, the optimized reaction conditions were utilised for the synthesis of 1-alkyl/aryl/dialkylamino-substituted isoquinolines 9a–f, when isoquinoline-N-oxide 8 was reacted with different alkyl/aryl/dialkyl amines at 0 °C to room temperature for 6–8 h in the presence of triflic anhydride and acetonitrile, as shown in Scheme 5 and Table 4.
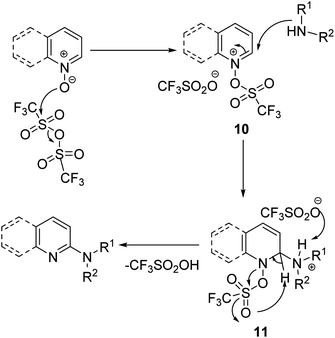
In the mechanistic step, triflic anhydride reacts with quinoline-N-oxide to produce the activated quinoline-N-oxide intermediate 10. Further, the activated quinoline-N-oxide intermediate 10 reacted with amine via nucleophilic addition to produce intermediate 11. The hydrogen of the ammonium intermediate 11 is abstracted by the trifluoromethane sulfonate anion, followed by aromatization to give the 2-amino-substituted quinoline (Scheme 6). Trifluoromethane sulfonic anhydride enhanced the CH-acidity and electrophilicity of the C-2 position by reacting with the N-oxide.
Conclusions
In conclusion, we have developed a straightforward and metal-free methodology for the regioselective amination of quinoline-N-oxides and isoquinoline-N-oxides with different aliphatic and aromatic amines utilising triflic anhydride as activator in a one-pot reaction. A wide range of 2-alkyl/aryl/dialkylamino-substituted quinolines and 1-alkyl/aryl/dialkylamino-substituted isoquinolines were synthesised in up to 84% yield. This amination exposed a good functional group tolerance and proceeds well when electron-donating and -withdrawing substituted amines were used.Experimental
General
Unless otherwise noted, all the reactions were performed in oven-dried glassware. The solvents used were dried and distilled. The reactions were performed under a nitrogen atmosphere. Acetonitrile was distilled from CaH2 and stored over 4 Å molecular sieves. The N-oxides and amines used were commercially available. All other commercial reagents were used without further purification, unless otherwise indicated. 1H NMR and 13C NMR spectra were recorded on 400 MHz and 101 MHz Bruker spectrometers, respectively, using either CDCl3 or DMSO-d6 as solvent, with tetramethylsilane (TMS) as internal standard.General experimental procedure
To a solution of quinoline-/isoquinoline-N-oxide (1.0 mmol, 1.0 equiv.) and amine (1.2 mmol, 1.2 equiv.) in CH3CN (8 mL) was added Tf2O (0.25 mL, 1.5 mmol, 1.5 equiv.) drop by drop at 0 °C. The reaction mixture was stirred for 6–8 h at room temperature and the reaction was monitored by thin layer chromatography. After completion of the reaction, the solvent was evaporated under vacuum, and the residue was quenched with saturated NaHCO3 solution (20 mL), and extracted with CH2Cl2 (3 × 50 mL). The combined organic layer was washed with brine (15 mL) and dried over anhydrous Na2SO4. The combined organic layer was concentrated and purified by column chromatography on silica gel (60–120 mesh) using a mixture of petroleum ether and ethylacetate as eluent to give pure product.4-(Quinolin-2-yl)morpholine, 5a14a
Yield 82% (175 mg); bone off-white solid; mp 88–89 °C; 1H NMR (400 MHz, CDCl3) δ: 7.85 (d, J = 9.1 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.54 (d, J = 1.1 Hz, 1H), 7.56–7.46 (m, 1H), 7.20–7.16 (m, 1H), 6.90 (d, J = 9.1 Hz, 1H), 3.79 (t, J = 4.8 Hz, 4H), 3.65 (t, J = 5.0 Hz, 4H); 13C NMR (101 MHz, CDCl3) δ: 157.6, 147.6, 137.6, 129.7, 127.3, 126.8, 123.3, 122.7, 109.3, 66.9, 45.6; HRMS (ESI) m/z calcd for C13H15N2O: 215.1184, found: 215.1182.2-(Piperidin-1-yl)quinoline, 5b16b
Yield 79% (167.0 mg); mp 46–47 °C; 1H NMR (400 MHz, CDCl3) δ: 8.41 (d, J = 9.2 Hz, 1H), 8.25 (d, J = 8.4 Hz, 1H), 8.13 (d, J = 9.2 Hz, 1H), 8.10–8.02 (m, 1H), 7.82–7.73 (m, 1H), 7.55 (d, J = 9.2 Hz, 1H), 4.31–4.26 (m, 4H), 2.25 (brs, 6H); 13C NMR (101 MHz, CDCl3) δ: 157.7, 148.0, 137.5, 129.3, 127.2, 126.5, 122.8, 121.8, 109.8, 46.3, 25.8, 24.8; HRMS (ESI) m/z calcd for C14H17N2: 213.1392, found: 213.1382.N-Butylquinolin-2-amine, 5c16b
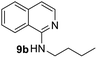
Yield 82% (164 mg); viscous liquid; 1H NMR (400 MHz, CDCl3) δ: 7.83 (d, J = 8.9 Hz, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.57–7.51 (m, 1H), 7.24–7.18 (m, 1H), 6.65 (d, J = 8.9 Hz, 1H), 4.76 (brs, 1H), 3.50 (q, J = 7.2 Hz, 2H), 1.77–1.55 (m, 2H), 1.53–1.44 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 157.2, 148.2, 137.3, 129.5, 127.5, 126.0, 123.4, 121.9, 111.2, 41.6, 31.9, 20.3, 13.9.N-Benzylquinolin-2-amine, 5d14c
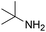
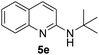
Yield 84% (196 mg); colourless crystalline solid; mp 97–98 °C; 1H NMR (400 MHz, CDCl3) δ: 7.84 (d, J = 8.8 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.62 (dd, J = 8.0, 1.1 Hz, 1H), 7.59–7.55 (m, 1H), 7.47–7.42 (m, 2H), 7.40–7.34 (m, 2H), 7.34–7.28 (m, 1H), 7.28–7.23 (m, 1H), 6.66 (d, J = 8.9 Hz, 1H), 5.06 (s, 1H), 4.76 (d, J = 5.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 156.8, 148.1, 139.5, 137.5, 129.7, 128.7, 127.9, 127.5, 127.4, 126.3, 123.6, 122.2, 111.5, 45.9; HRMS (ESI) m/z calcd for C16H15N2: 235.1235, found: 235.1240.N-(tert-Butyl)quinolin-2-amine, 5e18
Yield: 68% (136 mg); light yellow oil; 1H NMR (400 MHz, CDCl3) δ: 1.53 (s, 9H), 5.49 (brs, 1H), 6.65 (d, J = 9.0 Hz, 1H), 7.16–7.21 (m, 1H), 7.48–7.7.53 (m, 1H), 7.55 (dd, J = 8.0 Hz, 1.0 Hz, 1H), 7.77 (d, J = 9.0 Hz, 1H).N-Benzyl-N-methylquinolin-2-amine, 5f16c
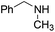
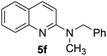
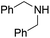
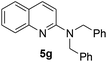
Yield 76% (188 mg); colourless crystalline solid; mp 94–95 °C; 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J = 8.9 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.63 (dd, J = 8.0 Hz, 1.3 Hz, 1H), 7.59–7.55 (m, 1H), 7.38–7.20 (m, 6H), 6.91 (d, J = 9.1 Hz, 1H), 4.98 (s, 2H), 3.26 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 157.3, 148.3, 138.7, 137.5, 129.6, 128.7, 127.4, 127.3, 127.2, 126.6, 122.8, 121.9, 109.1, 53.3, 36.3; HRMS (ESI) m/z calcd for C17H17N2: 249.1392, found: 249.1397.N,N-Dibenzylquinolin-2-amine, 5g14c
Yield 74% (240 mg); bone off-white solid; mp 101–102 °C; 1H NMR (400 MHz, CDCl3) δ: 7.85 (d, J = 9.1 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.65–7.54 (m, 2H), 7.38–7.20 (m, 11H), 6.85 (d, J = 9.1 Hz, 1H), 4.97 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 157.1, 148.2, 138.6, 137.7, 129.6, 128.7, 127.5, 127.3, 127.2, 126.8, 122.9, 122.0, 109.2, 50.8; HRMS (ESI) m/z calcd for C23H21N2: 325.1705, found: 325.1708.N-Phenylquinolin-2-amine, 5h14c
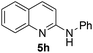
Yield 79% (174 mg); brown solid; mp 93–94 °C; 1H NMR (400 MHz, CDCl3) δ: 7.94 (d, J = 8.9 Hz, 1H), 7.79 (d, J = 8.9 Hz, 1H), 7.66 (dd, J = 8.0 Hz, 1.3 Hz, 1H), 7.64–7.59 (m, 1H), 7.57 (dd, J = 8.6 Hz, 1.1 Hz, 2H), 7.43–7.35 (m, 2H), 7.33–7.29 (m, 1H), 7.14–7.08 (m, 1H), 7.01 (d, J = 8.9 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 154.5, 147.0, 140.0, 138.2, 130.1, 129.3, 127.6, 126.2, 124.9, 124.0, 123.3, 120.9, 111.7; HRMS (ESI) m/z calcd for C15H13N2: 221.1079, found: 221.1071.N-(4-Bromophenyl)quinolin-2-amine 5i14c
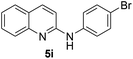
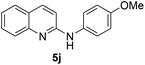
Yield 77% (230 mg); colourless crystalline solid; mp 146–147 °C; 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J = 8.9 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.65–7.58 (m, 3H), 7.49 (d, J = 8.9 Hz, 2H), 7.35 (t, J = 7.5 Hz, 1H), 6.93 (d, J = 8.9 Hz, 1H), 6.73 (s, 1H); 13C NMR (101 MHz, CDCl3) δ: 153.7, 147.4, 139.5, 137.9, 132.1, 129.8, 127.5, 126.9, 124.2, 123.5, 121.4, 115.0, 112.1.N-(4-Methoxyphenyl)quinolin-2-amine 5j14c
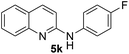
Yield 78% (195 mg); colourless crystalline solid; mp 125–126 °C; 1H NMR (400 MHz, CDCl3) δ: 7.89 (d, J = 8.9 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 9.0 Hz, 1H), 7.59 (t, J = 7.7 Hz, 1H), 7.44 (d, J = 8.9 Hz, 2H), 7.32–7.26 (m, 1H), 6.96–6.93 (m, 2H), 6.89 (d, J = 8.9 Hz, 1H), 6.79 (s, 1H), 3.85 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 156.3, 155.7, 147.8, 137.7, 133.2, 129.8, 127.5, 126.2, 123.9, 122.5, 114.6, 111.3, 55.4; HRMS (ESI) m/z calcd for C16H15N2O: 251.1184, found: 251.1173.N-(4-Fluorophenyl)quinolin-2-amine 5k14c
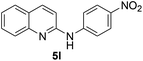
Yield 67% (159 mg); colourless crystalline solid; mp 101–103 °C; 1H NMR (400 MHz, CDCl3) δ: 7.85 (d, J = 8.9 Hz, 1H), 7.66 (d, J = 4.4 Hz, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.53–7.48 (m, 1H), 7.46–7.42 (m, 2H), 7.25–7.21 (m, 1H), 6.99 (t, J = 8.7 Hz, 2H), 6.75 (d, J = 8.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 159.3 (d, J = 252.5 Hz), 154.4, 146.4, 138.5, 135.7 (d, J = 2.7 Hz), 130.3, 127.6, 126.6 (d, J = 8.1 Hz), 125.7, 123.9, 123.5, 123.1 (d, J = 7.9 Hz), 116.0 (d, J = 22.5 Hz), 115.6 (d, J = 12.9 Hz), 111.5; HRMS (ESI) m/z calcd for C15H12FN2: 239.0985, found: 239.0990.N-(4-Nitrophenyl)quinolin-2-amine 5l17
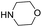
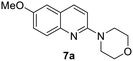
Yield 62% (164 mg); yellow solid; mp 202–203 °C; 1H NMR (400 MHz, CDCl3) δ: 8.80 (s, 1H), 8.28 (d, J = 8.8 Hz, 2H), 8.16 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.83–7.76 (m, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.38–7.28 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 150.9, 149.3, 145.3, 140.9, 130.3, 129.6, 126.1, 123.0, 121.8, 118.1, 107.5; HRMS (ESI) m/z calcd for C15H12N3O2: 266.0930, found: 266.0936.4-(6-Methoxyquinolin-2-yl)morpholine, 7a16b
Yield 83% (203 mg); colourless crystalline solid; mp 129–130 °C; 1H NMR (400 MHz, CDCl3) δ: 7.87 (d, J = 9.0 Hz, 1H), 7.68 (d, J = 9.1 Hz, 1H), 7.30–7.22 (m, 1H), 6.99–6.95 (m, 2H), 3.90 (s, 3H), 3.88 (t, J = 6.0 Hz, 4H), 3.66 (t, J = 6.0 Hz, 4H); 13C NMR (101 MHz, CDCl3) δ: 156.7, 155.3, 143.3, 136.6, 128.3, 123.8, 121.3, 109.7, 106.0, 66.9, 55.5, 45.9; HRMS (ESI) m/z calcd for C14H17N2O2: 245.1290, found: 245.1294.N-Butyl-6-methoxyquinolin-2-amine, 7b
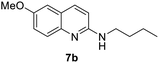
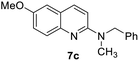
Yield 66% (152 mg); brown solid; mp 81–82 °C; 1H NMR (400 MHz, CDCl3) δ: 7.66 (d, J = 8.9 Hz, 1H), 7.52 (d, J = 9.1 Hz, 1H), 7.12 (dd, J = 9.1 Hz, 2.9 Hz, 1H), 6.86 (d, J = 2.8 Hz, 1H), 6.54 (d, J = 8.9 Hz, 1H), 4.56 (s, 1H), 3.78 (s, 3H), 3.35 (q, J = 8.0 Hz, 2H), 1.64–1.50 (m, 2H), 1.42–1.33 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 156.1, 154.7, 143.5, 136.4, 127.4, 123.6, 120.9, 111.2, 106.6, 55.5, 41.7, 32.0, 20.2, 13.9; HRMS (ESI) m/z calcd for C14H19N2O: 231.1497, found: 231.1493.N-Benzyl-6-methoxy-N-methylquinolin-2-amine, 7c
Yield 64% (178 mg); colourless crystalline solid; mp 93–95 °C; 1H NMR (400 MHz, CDCl3) δ: 7.81 (d, J = 9.1 Hz, 1H), 7.69 (d, J = 9.1 Hz, 1H), 7.40–7.21 (m, 6H), 6.98 (d, J = 2.8 Hz, 1H), 6.89 (d, J = 9.1 Hz, 1H), 4.93 (s, 2H), 3.90 (s, 3H), 3.23 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 156.3, 154.7, 143.7, 138.8, 136.5, 128.6, 127.9, 127.2, 127.0, 122.9, 121.1, 109.3, 106.2, 55.5, 53.4, 36.2; HRMS (ESI) m/z calcd for C18H19N2O: 279.1497, found: 279.1494.6-Methoxy-N-phenylquinolin-2-amine, 7d18
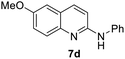
Yield 62% (155 mg); white powder; mp 145–146 °C; 1H NMR (400 MHz, CDCl3) δ: 7.86 (d, J = 8.9 Hz, 1H), 7.75 (d, J = 9.1 Hz, 1H), 7.60–7.52 (m, 2H), 7.42–7.33 (m, 2H), 7.30 (dd, J = 9.0 Hz, 2.8 Hz, 1H), 7.13–7.06 (m, 1H), 7.01 (t, J = 5.8 Hz, 2H), 6.86 (s, 1H), 3.91 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 155.6, 153.0, 143.2, 140.6, 136.7, 129.2, 128.2, 124.7, 122.7, 121.4, 120.0, 112.0, 106.3, 55.5; HRMS (ESI) m/z calcd for C16H15N2O: 251.1184, found: 251.1182.6-Methoxy-N-(4-methoxyphenyl)quinolin-2-amine, 7e19
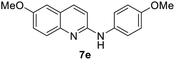
Yield 65% (182 mg); colourless crystalline solid; mp 146–147 °C; 1H NMR (400 MHz, CDCl3) δ: 7.82 (d, J = 8.9 Hz, 1H), 7.68 (d, J = 9.1 Hz, 1H), 7.42 (d, J = 8.9 Hz, 2H), 7.27 (dd, J = 9.1 Hz, 2.9 Hz, 1H), 7.00 (d, J = 2.8 Hz, 1H), 6.94 (d, J = 8.9 Hz, 2H), 6.89 (d, J = 8.9 Hz, 1H), 6.71 (s, 1H), 3.90 (s, 3H), 3.84 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 156.1, 155.3, 154.1, 143.3, 136.7, 133.5, 127.9, 124.4, 123.5, 121.3, 114.6, 111.2, 106.4, 55.6, 55.6; HRMS (ESI) m/z calcd for C17H17N2O2: 281.1290, found: 281.1291.6-Methoxy-N-(4-nitrophenyl)quinolin-2-amine, 7f
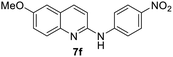
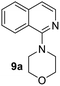
Yield 60% (183 mg); colourless crystalline solid; mp 218–219 °C; 1H NMR (400 MHz, DMSO-d6) δ: 9.50 (s, 1H), 8.58 (d, J = 5.0 Hz, 1H), 8.22 (d, J = 9.1 Hz, 2H), 7.92 (d, J = 9.2 Hz, 1H), 7.60 (d, J = 2.6 Hz, 1H), 7.50–7.32 (m, 4H), 3.93 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 157.5, 149.6, 148.6, 145.6, 143.9, 140.7, 131.5, 126.2, 122.8, 122.2, 117.8, 108.8, 101.6, 56.2; HRMS (ESI) m/z calcd for C16H14N3O3: 296.1035, found: 296.1038.4-(Isoquinolin-1-yl)morpholine, 9a16c
Yield 83% (177 mg); colourless crystalline solid; mp 67–68 °C; 1H NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 5.6 Hz, 1H), 7.96 (d, J = 8.4 Hz, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.47 (t, J = 7.0 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.12 (d, J = 5.7 Hz, 1H), 3.84 (t, J = 4.6 Hz, 4H), 3.28 (t, J = 4.4 Hz, 4H); 13C NMR (101 MHz, CDCl3) δ: 161.1, 140.7, 138.1, 129.7, 127.2, 126.2, 125.3, 121.6, 116.2, 67.1, 51.9; HRMS (ESI) m/z calcd for C13H15N2O: 215.1184, found: 215.1182.N-Butylisoquinolin-1-amine, 9b20a
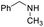
Yield 77% (154 mg); viscous liquid; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J = 5.9 Hz, 1H), 7.74 (d, J = 8.3 Hz, 1H), 7.63 (d, J = 7.9 Hz, 1H), 7.55 (dd, J = 7.0 Hz, 0.9 Hz, 1H), 7.48–7.34 (m, 1H), 6.90 (d, J = 5.8 Hz, 1H), 5.34 (s, 1H), 3.60 (t, J = 7.2 Hz, 2H), 1.78–1.63 (m, 2H), 1.49–1.42 (m, 2H), 0.97 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 155.3, 141.4, 137.0, 129.6, 127.1, 125.8, 121.4, 118.2, 110.6, 41.7, 31.7, 20.4, 14.0; HRMS (ESI) m/z calcd for C13H17N2: 201.1392, found: 201.1391.N-Benzyl-N-methylisoquinolin-1-amine, 9c20b
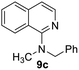
Yield 74% (184 mg); viscous liquid; 1H NMR (400 MHz, CDCl3) δ: 8.03 (t, J = 6.8 Hz, 2H), 7.62 (d, J = 8.1 Hz, 1H), 7.49–7.43 (m, 1H), 7.36 (d, J = 7.5 Hz, 2H), 7.33–7.24 (m, 3H), 7.19 (t, J = 7.3 Hz, 1H), 7.09 (d, J = 5.7 Hz, 1H), 4.52 (s, 2H), 2.90 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 161.8, 140.6, 138.8, 138.4, 129.6, 128.6, 127.7, 127.1, 127.1, 125.9, 125.6, 121.6, 115.1, 59.3, 40.1; HRMS (ESI) m/z calcd for C17H17N2: 249.1392, found: 249.1387.N-Phenylisoquinolin-1-amine, 9d18
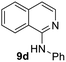
Yield 64% (141 mg); bone off-white solid, mp 111–112 °C; 1H NMR (400 MHz, CDCl3) δ: 8.17 (d, J = 5.7 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.76–7.73 (m, 3H), 7.65 (t, J = 7.5 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.41 (t, J = 7.9 Hz, 2H), 7.16 (d, J = 5.7 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 152.5, 140.9, 140.6, 137.5, 130.0, 129.0, 127.4, 126.5, 122.8, 121.7, 120.6, 119.0, 113.5; HRMS (ESI) m/z calcd for C15H13N2: 221.1079, found: 221.1074.N-(4-Methoxyphenyl)isoquinolin-1-amine, 9e19
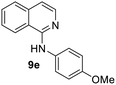
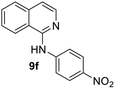
Yield 62% (155 mg); crystalline white solid; mp 129–130 °C; 1H NMR (400 MHz, CDCl3) δ: 8.08 (d, J = 5.8 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.75 (d, J = 8.1 Hz, 1H), 7.65 (t, J = 7.3 Hz, 1H), 7.60–7.45 (m, 3H), 7.10 (d, J = 5.8 Hz, 1H), 7.09 (s, 1H), 6.94 (d, J = 8.9 Hz, 2H), 3.83 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 155.8, 153.0, 141.1, 137.5, 133.4, 129.8, 127.4, 126.3, 123.2, 121.5, 118.6, 114.3, 112.8, 55.6; HRMS (ESI) m/z calcd for C16H15N2O: 251.1184, found: 251.1180.N-(4-Nitrophenyl)isoquinolin-1-amine, 9f
Yield 60% (159 mg); yellow solid; mp 219–120 °C; 1H NMR (400 MHz, CDCl3) δ: 8.26 (d J = 9.2 Hz, 2H), 8.22 (d J = 5.6 Hz, 1H), 8.00 (d, J = 8.3 Hz, 1H), 7.89 (d, J = 9.2 Hz, 2H), 7.86 (d, J = 8.0 Hz, 1H), 7.74 (t, J = 7.2 Hz, 1H), 7.66 (d, J = 7.2 Hz, 1H), 7.53 (s, 1H), 7.34 (d, J = 5.7 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 150.6, 146.7, 141.7, 140.5, 137.6, 130.4, 127.8, 127.3, 125.4, 121.1, 119.2, 118.0, 115.7; HRMS (ESI) m/z calcd for C15H12N3O2: 266.0930, found: 239.0936.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AKY and SVP acknowledge financial support from the UGC-FRP Start up grant (no. F.4-5/2017-2018) (Cycle-IV) (BSR) under UGC Faculty Recharge Programme UGC Govt. Of India.Notes and references
- (a) T. Tomioka, T. Takahashi and T. Maejima, Org. Biomol. Chem., 2012, 10, 5113–5118 RSC; (b) M. Miller, J. Shi, Y. Zhu, M. Kustov, J.-B. Tian, A. Stevens, M. Wu, J. Xu, S. Long, P. Yang, A. V. Zholos, J. M. Salovich, C. D. Weaver, C. R. Hopkins, C. W. Lindsley, O. McManus, M. Li and M. X. Zhu, J. Biol. Chem., 2011, 286, 33436–33446 CrossRef CAS PubMed.
- (a) P. P. Jumade, S. J. Wadher, A. J. Chourasia, U. V. Kharabe, D. Mude and P. G. Yeole, Int. J. Chem. Sci., 2009, 7, 1518–1530 CAS; (b) S. Al-Khalil, A. Alkofahi, D. El-Eisawi and A. Al-Shibib, J. Nat. Prod., 1998, 61, 262–263 CrossRef CAS PubMed; (c) R. Arienzo, D. E. Clark, S. Cramp, S. Daly, H. J. Dyke, P. Lockey, D. Norman, A. G. Roach, K. Stuttle, M. Tomlinson, M. Wong and S. P. Wren, Bioorg. Med. Chem. Lett., 2004, 14, 4099–4102 CrossRef CAS PubMed.
- (a) G. R. Proctor and A. L. Harvey, Curr. Med. Chem., 2000, 7, 295–302 CrossRef CAS PubMed; (b) Y. Cheng, T. C. Judd, M. D. Bartberger, J. Brown, K. Chen, R. T. Fremeau Jr, D. Hickman, S. A. Hitchcock, B. Jordan, V. Li, P. Lopez, S. W. Louie, Y. Luo, K. Michelsen, T. Nixey, T. S. Powers, C. Rattan, E. A. Sickmier, D. J. St. Jean Jr, R. C. Wahl, P. H. Wen and S. Wood, J. Med. Chem., 2011, 54, 5836–5857 CrossRef CAS PubMed.
- L. Strekowski, J. L. Mokrosz, V. A. Honkan, A. Czarny, M. T. Cegla, R. L. Wydra, S. E. Patterson and R. F. Schinazi, J. Med. Chem., 1991, 34, 1739–1746 CrossRef CAS PubMed and references cited therein.
- J. R. Pfister, J. Nat. Prod., 1988, 51, 969–970 CrossRef CAS PubMed.
- A. A. Alhaider, M. A. Abdelkader and E. J. Lien, J. Med. Chem., 1985, 28, 1394–1398 CrossRef CAS PubMed.
- S. F. Campbell, J. D. Hardstone and M. J. Palmer, J. Med. Chem., 1988, 31, 1031–1035 CrossRef CAS PubMed.
- (a) M. A. Solekhova and Yu. V. Kurbatov, Russ. J. Org. Chem., 2002, 38, 1192–1194 CrossRef CAS; (b) G. Li, C. Jia and K. Sun, Org. Lett., 2013, 15, 5198–5201 CrossRef CAS PubMed; (c) C. Zhu, M. Yi, D. Wei, X. Chen, Y. Wu and X. Cui, Org. Lett., 2014, 16, 1840–1843 CrossRef CAS PubMed; (d) Z. Wang, M.-Y. Han, P. Li and L. Wang, Eur. J. Org. Chem., 2018, 5954–5960 CrossRef CAS; (e) L.-Y. Xie, S. Peng, L.-L. Jiang, X. Peng, W. Xia, X. Yu, X.-X. Wang, Z. Caoc and W.-M. He, Org. Biomol. Chem., 2019, 17, 309–314 RSC; (f) L.-Y. Xie, S. Peng, L.-L. Jiang, X. Peng, W. Xia, X. Yu, X.-X. Wang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 6, 167–171 RSC.
- (a) N. G. Luthy, F. W. Bergstrom and H. S. Mosher, J. Am. Chem. Soc., 1949, 71, 1109–1110 CrossRef CAS PubMed; (b) J. Yin, B. Xiang, M. A. Huffman, C. E. Raab and I. W. Davies, J. Org. Chem., 2007, 72, 4554–4557 CrossRef CAS PubMed; (c) A. E. Chichibabin and O. A. Seide, Russ. J. Phys. Chem., 1914, 46, 1216–1236 CAS.
- Z. R. Wang, Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc., Hoboken, N. J., 2009 Search PubMed.
- (a) C. K. McGill and A. Rappa, Adv. Heterocycl. Chem., 1988, 44, 1–79 CrossRef CAS; (b) J. G. Rodriguez, C. de los Rios and A. Lafuente, Tetrahedron, 2005, 61, 9042–9051 CrossRef CAS; (c) D. Cuperly, P. Gros and Y. Fort, J. Org. Chem., 2002, 67, 238–241 CrossRef CAS PubMed; (d) J. Mathieu, P. Gros and Y. Fort, Chem. Commun., 2000, 951–952 RSC; (e) T. Imahori, M. Uchiyama, T. Sakamoto and Y. Kondo, Chem. Commun., 2001, 2450–2451 RSC.
- (a) A. T. Londregan, S. Jennings and L. Wei, Org. Lett., 2010, 12, 5254–5257 CrossRef CAS PubMed; (b) A. T. Londregan, S. Jennings and L. Wei, Org. Lett., 2011, 13, 1840–1843 CrossRef CAS PubMed.
- (a) B. W. Hansen and E. B. Pedersen, Liebigs Ann. Chem., 1981, 1485–1491 CrossRef; (b) J. Yin, B. Xiang, M. A. Huffman, C. E. Raab and I. W. Davies, J. Org. Chem., 2007, 72, 4554–4557 CrossRef CAS PubMed.
- (a) X. Chen, X. Li, Z. Qu, D. Ke, L. Qu, L. Duan, W. Mai, J. Yuan, J. Chen and Y. Zhao, Adv. Synth. Catal., 2014, 356, 1979–1985 CrossRef CAS; (b) W.-Z. Bi, K. Sun, C. Qu, X.-L. Chen, L.-B. Qu, S.-H. Zhu, X. Li, H.-T. Wu, L.-K. Duan and Y.-F. Zhao, Pure Appl. Chem., 2019, 91, 33–41 Search PubMed; (c) W.-Z. Bi, K. Sun, C. Qu, X.-L. Chen, L.-B. Qu, S.-H. Zhu, X. Li, H.-T. Wu, L.-K. Duan and Y.-F. Zhao, Org. Chem. Front., 2017, 4, 1595–1600 RSC.
- D. I. Bugaenko, M. A. Yurovskaya and A. V. Karchava, J. Org. Chem., 2017, 82, 2136–2149 CrossRef CAS PubMed.
- (a) Z. Yan, Z. Shiwei, X. Guangxing, L. Min, T. Chunlei and F. Weizheng, Org. Biomol. Chem., 2019, 17, 309–314 RSC; (b) H. Zhao, X. Chen, H. Jiang and M. Zhang, Org. Chem. Front., 2018, 5, 539–543 RSC; (c) Z. Hua, L. Fang, S. Wu and L. Wang, Eur. J. Org. Chem., 2016, 4953–4956 CrossRef CAS.
- H.-K. Peng, C.-K. Lin, S.-Y. Yang, C.-K. Tseng, C.-C. Tzeng, J.-C. Lee and S.-C. Yang, Bioorg. Med. Chem. Lett., 2012, 1107–1110 CrossRef CAS PubMed.
- W.-Z. Bi, K. Sun, C. Qu, X.-L. Chen, L.-B. Qu, S.-H. Zhu, X. Li, H.-T. Wu, L.-K. Duana and Y.-F. Zhao, Org. Chem. Front., 2017, 4, 1595–1600 RSC.
- A. K. Dhiman, D. Chandra, R. Kumar and U. Sharma, J. Org. Chem., 2019, 84, 6962–6969 CrossRef CAS PubMed.
- (a) X. Xie, T. Y. Zhang and Z. Zhang, J. Org. Chem., 2006, 71, 6522–6529 CrossRef CAS PubMed; (b) A. Ilie, G. -D. Roiban and M. T. Reetz, ChemistrySelect, 2017, 2, 1392–1397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: For further information of spectra, see the Supporting Information. See DOI: 10.1039/c9ra10397j |
| This journal is © The Royal Society of Chemistry 2020 |