Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly of pyrene-appended glucono gelators: spacer regulated morphological change and inversion of circularly polarized luminescence†
Zongwen Liuab,
Yuqian Jiangb,
Jian Jiang*b,
Chenhuan Yuanc,
Decai Wang*a and
Minghua Liu *bc
*bc
aCollege of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu, P. R. China. E-mail: dcwang998@126.com
bCAS Center for Excellence in Nanoscience, CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, Division of Nanophotonics, National Center for Nanoscience and Technology (NCNST), No. 11 ZhongGuanCun BeiYiTiao, Beijing 100190, P. R. China. E-mail: jiangj@nanoctr.cn; liumh@iccas.ac.cn
cBeijing National Laboratory for Molecular Science, CAS Key Laboratory of Colloid Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, No. 2 ZhongGuanCun BeiYiJie, Beijing 100190, P. R. China
First published on 13th February 2020
Abstract
Pyrene-appended glucono gelators with different spacer lengths (two and four methylene units) were designed and found to form supramolecular gels in organic aqueous solvents. The shorter spacer gelator 1 was prone to self-assemble into nanotubes due to well stacking multi-bilayer unit, while gelator 2 with the longer spacer formed nanofibers due to the relatively disordered packing structure. Both of the gels showed supramolecular chirality as well as circularly polarized luminescence (CPL) due to the chirality transfer from the glucose moiety to the assembly. Interestingly, the CD and CPL signals were opposite for the two gels. It was suggested that the packing of the pyrene unit in the gels were different due to the spacer and resulted in the inversed chiroptical properties. The work provided a deeper understanding of the origin of the supramolecular chirality and furthers the design of the CPL materials.
Self-assembly offers an efficient method to construct supramolecular gels, where small gelator molecules self-assembled into nanostructures and immobilized solvents via non-covalent interactions including hydrophobic interaction, hydrogen bonding, π–π stacking, electrostatic interaction, van der Waals forces and charge–transfer interactions.1–5 Interestingly, many of the chiral molecules easily form supramolecular gels meaning that the gels can be applied in chiral recognition, chiral separation, asymmetric catalysis and chiroptical functional materials.6–16
Circularly polarized luminescence (CPL) is a unique function that pertains to chiral systems, which emit different left- and right-handed circularly polarized light and have attracted great interest in recent years.16–22 By combining the chiral units and fluorophores, many of the chiral molecular self-assembly systems have been developed and efficient chiroptical CPL have been realized.18–22
In general, molecular systems show only one direction of CPL, this is determined by the molecular chirality of the chiral moiety. However, at a supramolecular level, supramolecular chirality can be controllably changed via regulating the self-assembly process or slightly modifying the molecules while maintaining the molecular chirality. For example, DNA could have right-handed B-DNA and left-handed Z-DNA was formed while originating from the same molecular chirality of D-sugar.23 In an artificial system, supramolecular chirality was also observed to be reversed by external stimulus. For example, the opposite CD spectra can be achieved through changing solvent, temperature, light irradiation, and addition of metal ions or achiral dopants.24–28 However, there were only limited cases that realize the inversion of CPL in supramolecular gels by tuning the self-assembly process.29–32 It still remains unknown why such chirality inversion could happen in supramolecular systems.
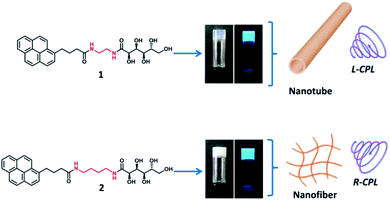
Here we designed two pyrene based derivatives (1–2, Fig. 1), in which a pyrene core served as an emission moiety, and a glucono group served as chiral source. The two parts were covalently connected via two amide bonds with different lengths of alkyl spacers. These molecules (1–2) can form supramolecular gels in the organic aqueous media. The self-assembled behaviour and luminescent property of the gel could be tuned dependent on the solvents and length of alkyl spacer chains, the nanotubes and nanofibers together with opposite CD and CPL signals were observed based on the different length of space alkyl chain in the gelator, as illustrated in Fig. 1.
4-(1-Pyrenyl)butyric acid methyl ester was employed as staring material, which was first reacted with ethylenediamine and 1,4-butanediamine respectively, the intermediate was separated and purified by gel column chromatography, and the desired gelator 1–2 was obtained after the intermediates were further reacted with glucono δ-lactone (ESI†).
The gelation behaviour of molecule 1–2 was tested in various solvents at a standard concentration with 1% (w/v). The results were summarized in Table S1,† both gelator 1 and gelator 2 were insoluble in a series of polar and nonpolar organic solvents including petroleum ether, dichloromethane, n-hexane, cyclohexane, acetone, ethyl acetate, THF, acetonitrile, methanol and ethanol (Table S1,† entry 1–10), and complex 1–2 were soluble in DMF and DMSO (Table S1,† entry 11 and 12). To our delicious, the compound 1–2 can form gel in the organic-water mixture solutions, including THF–H2O, DMSO–H2O and EtOH–H2O. In the case of ethanol water mixture solvent, the molecule 1 and 2 was first dissolved in ethanol–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture solution (Table S1,† entry 13). Further improving the content of water, the gel was formed (Table S1,† entry 14–16). Especially, when the volume ratio of EtOH
1) mixture solution (Table S1,† entry 13). Further improving the content of water, the gel was formed (Table S1,† entry 14–16). Especially, when the volume ratio of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O equal to 1
H2O equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, gelator 1 formed nearly transparent gel with critical gelation concentration (CGC) about 3 mg mL−1, while gelator 2 formed translucent gel with the CGC about 5 mg mL−1 (Fig. 1, photographs). In addition, the white gels were observed in the pure water (Table S1,† entry 17). Furthermore, the complex 1–2 exhibited similar gel behaviour in THF and DMSO aqueous solution as in ethanol–water solution (Table S1,† entry 18–22).
2, gelator 1 formed nearly transparent gel with critical gelation concentration (CGC) about 3 mg mL−1, while gelator 2 formed translucent gel with the CGC about 5 mg mL−1 (Fig. 1, photographs). In addition, the white gels were observed in the pure water (Table S1,† entry 17). Furthermore, the complex 1–2 exhibited similar gel behaviour in THF and DMSO aqueous solution as in ethanol–water solution (Table S1,† entry 18–22).
The morphology of 1 and 2 based gel in ethanol aqueous solution was first investigated (the gels were named as gel1 and gel2 corresponding to gelator 1 and gelator 2). It was found that the morphology of gel1 have obvious solvent dependent behaviour, scanning electron microscope (SEM) images shown that gel1 underwent change from nanofibers to nanorods and further to amorphous nanosheets in ethanol aqueous solution when gradually increasing the water content (Fig. S1†). It should be noted that uniform nanofibers were observed when gel1 formed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol
2 of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture solvent, the diameter distribution of nanofibers is extremely narrow (15 nm ± 1 nm), the length of nanofibers reached tens to hundred micrometers (Fig. 2a and S2†). TEM image indicated that these nanofibers were actually nanotubes, in which the diameter of nanotube was about 15 nm and the wall thickness of nanotube was around 2.5–2.7 nm (Fig. 2b and S2†). AFM image further verified that the height of the nanotube was around 15 nm (Fig. 2c and S3†). In the case of gel2, the nanofibers with the diameter around a few nanometers to tens of nanometers were observed in ethanol aqueous solution (Fig. S4†). Also, the size distribution of nanofibers was relatively uniform at 1
H2O mixture solvent, the diameter distribution of nanofibers is extremely narrow (15 nm ± 1 nm), the length of nanofibers reached tens to hundred micrometers (Fig. 2a and S2†). TEM image indicated that these nanofibers were actually nanotubes, in which the diameter of nanotube was about 15 nm and the wall thickness of nanotube was around 2.5–2.7 nm (Fig. 2b and S2†). AFM image further verified that the height of the nanotube was around 15 nm (Fig. 2c and S3†). In the case of gel2, the nanofibers with the diameter around a few nanometers to tens of nanometers were observed in ethanol aqueous solution (Fig. S4†). Also, the size distribution of nanofibers was relatively uniform at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol water solution (Fig. 2d). TEM and AFM images further proved that gel2 was nanofiber structure with the diameter of nanofiber around 5–10 nm (Fig. 2e, f and S5†). These results indicated that assembly behaviour of gelator 1 and 2 formed well defined nanostructures under 1
2 of ethanol water solution (Fig. 2d). TEM and AFM images further proved that gel2 was nanofiber structure with the diameter of nanofiber around 5–10 nm (Fig. 2e, f and S5†). These results indicated that assembly behaviour of gelator 1 and 2 formed well defined nanostructures under 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethanol water mixed solvent.
2 ethanol water mixed solvent.
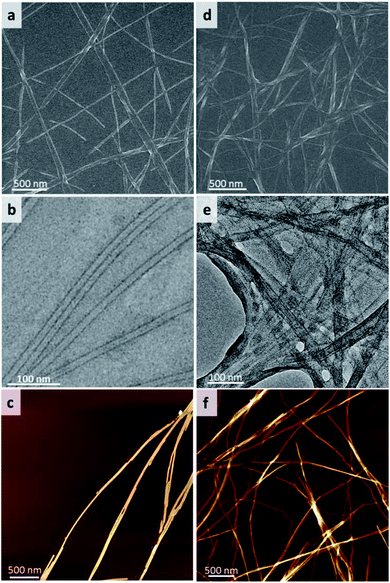 | ||
Fig. 2 SEM images of (a) gel1 and (d) gel2, TEM images of (b) gel1, (e) gel2 and AFM of images of (c) gel1 and (f) gel2. The gels were formed in mixed solvent of ethanol and water (EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
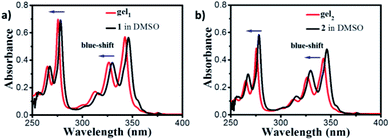
UV-vis spectra showed four main peaks at 266 nm, 277 nm, 328 and 345 nm of gelator 1 in DMSO dilute solution (Fig. 3a, black line, 1 × 10−5M), which can be assigned to the un-aggregated form of pyrene units (Fig. S6,† SEM and TEM of gelator 1 and 2 in DMSO indicated no assembly happened). The absorption peaks are blue shifted to 265 nm, 275 nm, 326 nm and 343 nm for gelator 1 in gel state (Fig. 3a, red line, gel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol
2 of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water). The blue-shifted bands were also observed in gel2 in comparison with 2 in DMSO diluted solution (Fig. 3b), these results suggested that in both cases, the gelator 1–2 prone to form H-aggregates in the gel state. Furthermore, it was found that the UV-vis spectra of gel1 and gel2 in different volume ratio of ethanol and water were nearly same as in 1
water). The blue-shifted bands were also observed in gel2 in comparison with 2 in DMSO diluted solution (Fig. 3b), these results suggested that in both cases, the gelator 1–2 prone to form H-aggregates in the gel state. Furthermore, it was found that the UV-vis spectra of gel1 and gel2 in different volume ratio of ethanol and water were nearly same as in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol
2 of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution (Fig. S7†).
water solution (Fig. S7†).
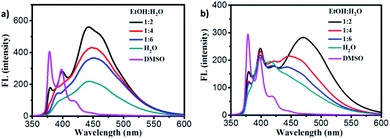
For exploring the emission properties of 1–2 gels, the fluorescence spectra were measured. In the diluted DMSO solution, two emission peaks at 380 nm and 396 nm ascribed to the monomer emission together with a very weak peak at 480 nm belong to excimer emission were observed for gelator 1 (Fig. 4a, purple line). In the gel1, the monomer emission peak decreased and excimer peak increased significantly, and the strong excimer emission peak at 442 nm was observed for the gel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol
2 of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture solution (Fig. 4a, black line). This phenomenon was also observed in the case of gel2, and found that the strong excimer peak at and 470 nm was observed (Fig. 4b, black line, gel in 1
water mixture solution (Fig. 4a, black line). This phenomenon was also observed in the case of gel2, and found that the strong excimer peak at and 470 nm was observed (Fig. 4b, black line, gel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of ethanol
2 of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water). The fluorescence quantum yields of gel1 and gel2 was about 28.2% and 9.4% respectively. Obviously, these results indicated that both gel1 and gel2 have strong π–π interaction between pyrene moieties.
water). The fluorescence quantum yields of gel1 and gel2 was about 28.2% and 9.4% respectively. Obviously, these results indicated that both gel1 and gel2 have strong π–π interaction between pyrene moieties.
Since the glucono group is chiral, the chiroptical properties of gels were further explored. There revealed several interesting features. First, when the gelator molecules 1–2 were dissolved into DMSO solution as a molecular state, no CD spectra can be detected in the absorption band of pyrene (Fig. S8†). However, obvious CD signals were observed when compounds 1–2 formed gels in mixed ethanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solvent. The negative CD signal of gel1 with the peaks at 294 nm, 366 nm and 382 nm were observed (Fig. 5a), which were consistency of corresponding to the UV-vis spectra of gel1, indicated that pyrene segments oriented in the chiral manner in the gel state. In the case of gel2, to our surprise, the positive CD signal with the peak at 290 nm, 344 nm and 367 nm were observed. It should be noted that gel2 presented opposite CD signal in comparison with gel1 in the longer wavelength regions. These results indicated that chiral packing manners of chromophore pyrene units in the gel state could be different, where the gelator spacer may regulate the supramolecular chirality of resulted gel assemblies. For quantitatively discuss comparison the supramolecular chirality, the dissymmetry factor of ECD, gCD value were found to be 9 × 10−4 and 1.4 × 10−4 for gel1 and gel2 respectively, indicated that gel1 with more uniform nanotube structure gave a stronger gCD. The CD signal based on the pyrene moiety was due to the chirality transfer from the glucose. Since in gel1, the distance between the pyrene and chiral unit is shorter, it is more efficient to transfer the chirality, thus leading to a larger gCD In addition, the CD spectra of gel1 and gel2 under different volume ratio of ethanol and water were also measured, and found that all of gel1 samples under different ethanol aqueous solution present negative CD, all of gel2 samples under different ethanol aqueous solution present positive CD (Fig. S9†), proved that the supramolecular chirality of both gel1 and gel2 was stable toward ethanol aqueous solvents.
2) solvent. The negative CD signal of gel1 with the peaks at 294 nm, 366 nm and 382 nm were observed (Fig. 5a), which were consistency of corresponding to the UV-vis spectra of gel1, indicated that pyrene segments oriented in the chiral manner in the gel state. In the case of gel2, to our surprise, the positive CD signal with the peak at 290 nm, 344 nm and 367 nm were observed. It should be noted that gel2 presented opposite CD signal in comparison with gel1 in the longer wavelength regions. These results indicated that chiral packing manners of chromophore pyrene units in the gel state could be different, where the gelator spacer may regulate the supramolecular chirality of resulted gel assemblies. For quantitatively discuss comparison the supramolecular chirality, the dissymmetry factor of ECD, gCD value were found to be 9 × 10−4 and 1.4 × 10−4 for gel1 and gel2 respectively, indicated that gel1 with more uniform nanotube structure gave a stronger gCD. The CD signal based on the pyrene moiety was due to the chirality transfer from the glucose. Since in gel1, the distance between the pyrene and chiral unit is shorter, it is more efficient to transfer the chirality, thus leading to a larger gCD In addition, the CD spectra of gel1 and gel2 under different volume ratio of ethanol and water were also measured, and found that all of gel1 samples under different ethanol aqueous solution present negative CD, all of gel2 samples under different ethanol aqueous solution present positive CD (Fig. S9†), proved that the supramolecular chirality of both gel1 and gel2 was stable toward ethanol aqueous solvents.
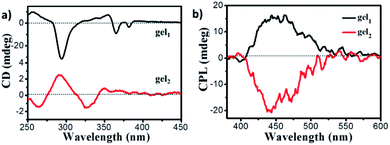 | ||
Fig. 5 (a) CD spectra of gel1 (black line) and gel2 (red line); (b) CPL of gel1 (black line) and gel2 (red line) (gelled in EtOH/H2O mixture solution, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), excited at 320 nm. 2), excited at 320 nm. | ||
Since the gel is fluorescent, the CPL spectra of gels were further investigated. It was found that both of gels exhibited CPL signals. The gel1 exhibited CPL signal with a maximum emission peak at 445 nm, which was coincided with the fluorescent excimer emission peaks (Fig. 5b). However, no obvious CPL can be detected when gelator 1 dissolved in DMSO (Fig. S10†), confirmed that CPL was originated from the supramolecular chiral aggregation of fluorescent pyrene units in the gel. The luminescence dissymmetry factor, glum = 2 × (IL − IR)/(IL + IR) was used to characterize CPL performance, where IL and IR are the luminescence intensities of right and left circularly polarized light respectively. The glum of gel1 is about 2.5 × 10−3. As expected, gel2 exhibited a nearly completely opposite CPL signal in comparison to gel1, the glum of which is about −3.3 × 10−3. Therefore, it was confirmed that supramolecular chiral alignment of pyrene fragments in the gel is able to emit the CPL, while changing the length of space in the gelator can inverse CPL. In addition, the CPL of gel1 and gel2 under different volume ratio of ethanol and water were also investigated, and found that the highest glum was found at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethanol and water solvent both for gel1 and gel2 (Fig. S11†), indicated that ordered assembly architectures gave a higher CPL intensity.
2 ethanol and water solvent both for gel1 and gel2 (Fig. S11†), indicated that ordered assembly architectures gave a higher CPL intensity.
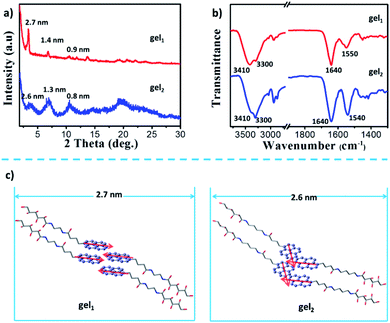
X-ray diffraction (XRD) was employed for exploring the molecular pacing in xerogels. As shown in Fig. 6a, gel1 showed the peaks with d-spacing of 2.7, 1.4 and 0.90 nm based on the Braggs eqn, indicating a lamellar packing structure according to the ratio of 1, 1/2 and 1/3. The d space of lamellar structure is about 2.7 nm, which is close to the wall thickness of nanotube observed by TEM. In the case of the gel2, the gelators also self-assembled into the lamellar structure with d spacing of 2.6 nm according to the diffraction peaks, suggesting that nanofibers consist of gel2 was about two to multi lamellar thickness.
FT-IR spectrum was employed to provide the interaction modes in the gels. Two strong vibrations were observed at 3410 and 3300 cm−1, which could be assigned to O–H and N–H vibrations, respectively. These two bands were from the glucone unit and the amide band, respectively. From the wavenumbers of the vibrations, it is clear that these gelators molecules formed H-bond between the glucone and amide groups. In addition, in both of gel state, the band at 1640 cm−1 were observed, which was assigned to the vibration of amide I. However, the different in the position and hydrogen bonding interaction showed characteristic peaks for a hydrogen bonded amide II, the stretching bonds at 1550 cm−1 was belonged to gel1, and gel2 shown the vibration band at 1540 cm−1 (Fig. 6b), indicated the hydrogen-bonding interaction between gelators in gel2 was weaker than that in gel1.
Based on these data, a possible packing model of gel1 and gel2 was proposed. In both of the gel systems, the molecules packed in a basic bilayer mode, like many of the amphiphiles. Here, the glucone group are in the outside while the pyrene packed inside the bilayer. Such bilayers were stabilized by the intermolecular H-bond between amide and the glucono unit, as shown in the FT-IT spectra. Furthermore, the strong excimer peak was observed, indicating that π–π interactions existed in both gel systems. It should be noted the π–π conjugated degree between pyrene units of gel2 may be different, which is regulated by the spacers. It should be noted that gelator 2 has a longer spacer length with four methylene units, while gelator 1 has only two methylene units. However, the d spacer from the XRD is longer for gel1 (2.7 nm), while shorter for gel2. This indicated that in gel2, the molecules are more tilted oriented. A possible orientation could be illustrated as in Fig. 6c. In the case of gel1, pyrene packed parallel to each other and the bilayer showed ordered structures. Due to the ordered packing, the bilayer rolled into nanotube and showed a stronger excimer emission. Due to the well-packing in an H-aggregate, the excimer appeared in a relatively shorter wavelength. In the gel2, due to the longer spacer and flexibility, the pyrene packed relatively disordered. It seems that the pyrene can interpenetrate between each layers and caused partial overlap stacking of the pyrene unit, which can be seen from the Cotton effect in the case of gel2, where a split existed around 250–300 nm, belonging to the shorter axis of the pyrene. Thus, in gel1, the dipole moment of the pyrene is parallel, while in gel2, those of the dipole moments constituted a larger angle, as indicated in Fig. 4c. According the exciton theory, these two alignments would cause the opposite CD signals. Thus, we observed the opposited CD signals in gel1 and gel2. In the excited state, it seemed that there is no big change of the conformation, thus, we observed also the opposite CPL. Thus, through the spacer regulation, we can realized the inversion of CD and CPL signals, which provided a deep insight into the origin of the supramolecular chirality and regulating method.
In conclusion, two pyrene based gelators were designed and found to form gels in the mixed solvents of water and ethanol. Due to the hydrogen bond between amide and glucone and the π–π stacking of the pyrene, lamellar structures were formed as a basic unit. The shorted spacer of gel1 caused the well-defined π–π stacking of the pyrene and rolled into nanotube. In gel2, a well-packed bilayer unit and relatively disordered packing of pyrene lead to the nanofiber formation. The molecular chirality of the glucone unit can transfer to the assemblies that lead to the CD and CPL of the nanotube and nanofiber. Interestingly, the nanotube and the nanofiber showed the opposite CD and CPL signals, which was suggested to be due to the different packing of the pyrene units in the lamellar structures. Thus, the work provided a new understanding of the origin of the supramolecular chirality and a way to regulate the chiral self-assembly and the chiroptical via spacer length.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by National Natural Science Foundation of China (21861132002, 21773043 and 21973020) and fund of the Chinese Academy of Sciences. Key Research Program of Frontier Sciences, CAS, (QYZDJ-SSW-SLH044).Notes and references
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS.
- P. Xing and Y. Zhao, Acc. Chem. Res., 2018, 51, 2324–2334 CrossRef CAS.
- Y. Ma, M. Cametti, Z. Džlic and S. Jiang, J. Mater. Chem. C, 2016, 4, 10786 RSC.
- M. Liu, G. Ouyang, D. Niu and Y. Sang, Org. Chem. Front., 2018, 5, 2885–2900 RSC.
- Y. Lan, M. G. Corradini, R. G. Weiss, S. R. Raghavan and M. A. Rogers, Chem. Soc. Rev., 2015, 44, 6035–6058 RSC.
- D. Gambhir, S. Kumar, G. Dey, V. Krishnan and R. R. Koner, Chem. Commun., 2018, 54, 11407–11410 RSC.
- T. Tu, W. Fang, X. Bao, X. Li and K. H. Doetz, Angew. Chem., Int. Ed., 2011, 50, 6601–6605 CrossRef CAS.
- X. Xu, L. Qu, J. Song, D. Wu, X. Zhou and H. Xiang, Chem. Commun., 2019, 55, 9873–9876 RSC.
- D. Gambhir, S. Kumar, G. Dey, V. Krishnan and P. R. Koner Liu, Chem. Commun., 2018, 54, 11407–11410 RSC.
- L. Zhang, Q. Jin and M. Liu, Chem.–Asian J., 2016, 11, 2642–2649 CrossRef CAS.
- W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2014, 136, 1116–1124 CrossRef CAS.
- C. Zhou, Y. Ren, J. Han, Q. Xu and R. Guo, ACS Nano, 2019, 13, 3534–3544 CrossRef CAS PubMed.
- J. Jiang, Y. Meng, L. Zhang and M. Liu, J. Am. Chem. Soc., 2016, 138, 15629–15635 CrossRef CAS PubMed.
- C. Yuan, J. Jiang, H. Sun, D. Wang, Y. Hu and M. Liu, Chemcatchem, 2018, 10, 2190–2194 CrossRef CAS.
- J. Jiang, G. Ouyang, L. Zhang and M. Liu, Chem. - Eur. J., 2017, 23, 9439–9450 CrossRef CAS PubMed.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2019 DOI:10.1002/adma.201900110.
- L. Wang, L. Yin, W. Zhang, X. Zhu and M. Fujiki, J. Am. Chem. Soc., 2017, 139, 13218–13226 CrossRef CAS PubMed.
- H. Zheng, W. Li, W. Li, X. Wang, Z. Tang, S. Zhang and Y. Xu, Adv. Mater., 2018, 30, 1705948 CrossRef PubMed.
- X. Zhang, Y. Zhang, Y. Li, Y. Quan, Y. Cheng and Y. Li, Chem. Commun., 2019, 55, 9845–9848 RSC.
- M. Li, Si. Li, D. Zhang, M. Cai, L. Duan, M. Fung and C. Chen, Angew. Chem., Int. Ed., 2018, 57, 2889–2893 CrossRef CAS.
- Z. Sun, J. Liu, D. Yuan, Z. Zhao, X. Zhu, D. Liu, Q. Peng and C. Zhao, Angew. Chem., Int. Ed., 2019, 58, 4840–4846 CrossRef CAS PubMed.
- S. Liu, F. Li, Y. Wang, X. Li, C. Zhu and Y. Cheng, J. Mater. Chem. C, 2017, 5, 6030–6036 RSC.
- M. Fuertes, V. Cepeda, C. Alonso and J. Pérez, Chem. Rev., 2006, 106, 2045–2064 CrossRef CAS PubMed.
- D. Zhao, T. van Leeuwen, J. Cheng and B. L. Feringa, Nat. Chem., 2017, 9, 250–256 CrossRef CAS PubMed.
- G.-F. Liu, L.-Y. Zhu, W. Ji, C.-L. Feng and Z.-X. Wei, Angew. Chem., Int. Ed., 2016, 55, 2411–2415 CrossRef CAS PubMed.
- F. Aparicio, B. Nieto-Ortega, F. Najera, F. J. Ramirez, J. T. L. Navarrete, J. Casado and L. Sanchez, Angew. Chem., Int. Ed., 2014, 53, 1373–1377 CrossRef CAS PubMed.
- S. Arias, F. Freire, E. Quinoa and R. Riguera, Angew. Chem., Int. Ed., 2014, 53, 13720–13724 CrossRef CAS PubMed.
- C. D. Jones, H. T. D. Simmons, K. E. Horner, K. Liu, R. L. Thompson and J. W. Steed, Nat. Chem., 2019, 11, 375–381 CrossRef CAS PubMed.
- H. Anetai, T. Takeda, N. Hoshino, Y. Arak, T. Wada, S. Yamamoto, M. Mitsuishi, H. Tsuchida, T. Ogoshi and T. Akutagawa, J. Phys. Chem. C, 2018, 122, 6323–6331 CrossRef CAS.
- P. Li, B. Lue, D. Han, P. Duan, M. Liu and M. Yin, Chem. Commun., 2019, 55, 2194–2197 RSC.
- D. Niu, Y. Jiang, L. Ji, G. Ouyang and M. Liu, Angew. Chem., Int. Ed., 2019, 58, 5946–5950 CrossRef CAS PubMed.
- F. Wang, W. Ji, P. Yang and C.-L. Feng, ACS Nano, 2019, 13, 7281–7290 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10315e |
| This journal is © The Royal Society of Chemistry 2020 |