Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal (Cd, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone-coated magnetite nanoparticles†
Jie Hongab,
Junyu Xiec,
Seyyedali Mirshahghassemib and
Jamie Lead*b
aCollege of Environment, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, China
bCenter for Environmental Nanoscience and Risk, Department of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, Columbia, SC 29208, USA. E-mail: jlead@mailbox.sc.edu
cCollege of Resources and Environment, Shanxi Agricultural University, Taigu, Shanxi 030801, China
First published on 20th January 2020
Abstract
Water pollution is a major global challenge given the increasing growth in industry and human population, and certain metals can be highly toxic and contribute to this significantly. In this study, polyvinylpyrrolidone-coated magnetic nanoparticles (PVP–Fe3O4 NPs) were used to remove metals (Cd, Cr, Ni, and Pb) from synthetic soft water and sea water in the presence and absence of fulvic acid. Nanoparticle (NP) suspensions were added to water media at a range of metal concentrations (0.1–100 mg L−1). Removal at different time points (1.5, 3, 6, 12, 24 hours) was also evaluated. Results showed that 167 mg L−1 PVP–Fe3O4 NPs could remove nearly 100% of four metals at 0.1 mg L−1 and more than 80% at 1 mg L−1. The removal decreased as the initial metal concentration increased, although essentially 100% of the Pb was removed under all conditions. The kinetic adsorption fitted well to the pseudo-second-order model and in general, the majority of metal adsorption occurred within the first 1.5 hours. These NPs are a reliable method to remove metals under a wide range of environmentally relevant conditions. Our previous research showed the NPs effectively removed oil from waters, so these NPs offer the possibility of combined in situ remediation of oil and metals.
1. Introduction
Metals, such as cadmium (Cd), chromium (Cr), nickel (Ni) and lead (Pb) are potentially highly toxic. These metals have a number of adverse outcomes for human health, including kidney failure, softening of bones, prostate cancer, and damage to the liver, children's central nervous system and the reproductive system.1–4 These metals are a potential risk, given these hazards and their wide exposure in the environment including their bioaccumulation and bio-magnification.5,6 The United States Environmental Protection Agency (USEPA) has set the maximum contaminant level (MCL), which is the highest level of a contaminant that is allowed in drinking water, at 0.005 mg L−1 for Cd, 0.1 mg L−1 for Cr, 0.1 mg L−1 for Ni and 0.015 mg L−1 for Pb.7 Recently, lead contamination (Pb level was 13–800 times higher than the EPA's MCL) in the City of Flint, MI caused serious environmental health issues.8 For soil exposure, our previous work has suggested that MCLs are not sufficiently protective for lead level in soil.9 We found a threshold value for low birth weight is about 130.5 mg kg−1 Pb in soil, which is lower than the EPA's hazardous value of 400 mg kg−1.10To date, many technologies have been used for metal-contaminated sites, such as stabilization, solidification, soil flushing, chemical reduction/oxidation, electrokinetics, low temperature thermal desorption, incineration, excavation/retrieval, disposal and landfill.11 However, these technologies are typically expensive and destructive. Phytoremediation may be a better option and less perturbing, but can be time consuming and disposal of the metal-contaminated plant material can be problematic.12 All available options therefore have benefits but also limitations and the need for new methods is pressing.
Recently, nanotechnology has been shown to provide a potentially cheap and effective solution for environmental remediation.13 Reactive nanomaterials such as nanoscale zeolites, metal oxides, carbon nanotubes, and bimetallic nanoparticles have been used for metal remediation.14 Iron oxide nanoparticles are widely used for metal remediation due to their low toxicity and easy separation from water media;15,16 in addition, where the NP is composed of magnetite, a facile magnetic separation of NPs, along with associated contaminants, can be performed. However, bare magnetite nanoparticles rapidly aggregate in aqueous systems and are highly susceptible to transformations under many environmental conditions,17,18 necessitating the use of appropriate capping agents. For instance, Fe3O4@SiO2 magnetic nanoparticles coated with poly(1-vinylimidazole) oligomer have been used to remove Hg(II) from water,19 while carbon-encapsulated nano-magnets have been used to extract metals even in acidic solutions.20 Ferromagnetic carbon-coated Fe nanoparticles have been use to remove nearly 95% Cr(VI) from aqueous solution,21 while nanoparticles with a magnetic core and a porous carbon shell could remove metals in acidic suspensions, with high efficiency through electrostatic attraction and adsorption.22
Previous work by the authors has shown that polyvinylpyrrolidone coated nanoparticles have great stability under relevant environmental conditions and that PVP is largely non-toxic.23–25 A new, facile and cost-effective hydrothermal synthesis technique, using no organic solvents and low temperature/energy requirements and low toxicity reactants was developed to produce PVP-coated Fe3O4 NPs.26–28 It has been shown that these synthesized NPs have a large capacity to remediate oil under environmentally relevant conditions.26,27 Here we extend their use to metal remediation (Cd, Cr, Ni, Pb) in the different synthetic water media under various conditions. Metal removal efficiency was evaluated under realistic conditions and the kinetics of adsorption of metals ions was quantified.
2. Methodology
2.1 Chemicals/materials
Polyvinylpyrrolidone (Mw 10 kDa), cadmium nitrate (Cd(NO3)2·4H2O, 99%), lead nitrate (Pb(NO3)2, 99%), nickel nitrate (Ni(NO3)2·6H2O, 99%), potassium dichromate (K2CrO7, 99%) were purchased from Sigma-Aldrich. FeCl3·6H2O (>98%) and ammonium hydroxide (NH4OH, 25–30%) were purchased from BDH and FeCl2·4H2O (98%) from Alfa Aesar. All chemicals were used as received without further purification.2.2 Preparation and characterization of PVP–Fe3O4 NPs
The PVP–Fe3O4 NPs synthesis used the method published before.26 Firstly, 28.8 mM of PVP was added to 6.25 mL ultrapure water (UHP, maximum resistivity 18.2 MΩ cm) while the solution was stirred at 80 ± 5 °C. Subsequently, 160 mM FeCl2·4H2O and 640 mM FeCl3·6H2O were added to the solution while the solution was stirred and the temperature was kept constant. Next, 19.2 mM PVP was dissolved in the solution. Finally, 6.25 mL ammonium hydroxide was added into the solution dropwise with vigorous stirring and the solution was mixed for 25 minutes at 90 ± 5 °C and then taken off the heat. After the precipitates reached room temperature, they were washed once with ultrapure water and separated using a 1.5 in. cubic neodymium magnet (Grade N 52, K&J Magnetics Inc.) and redispersed in ultrapure water by sonication. Characterization of the NPs was performed by atomic force microscopy (AFM), Fourier transform infrared spectrometer (FTIR), dynamic light scattering (DLS) and capillary electrophoresis.2.3 Metals adsorption analysis
For all experiments, NP concentrations of 167 mg L−1 were used. Suspensions were sonicated for 30 min and shaken (200 rpm) at 25 °C for different time periods. PVP–Fe3O4 NPs were then separated by a cubic magnet (Grade N 52, K&J magnetic Inc.) until the NPs were completely separated from the aqueous phase. The supernatant was then collected for metal element analysis by inductively coupled plasma optical emission spectrometry (ICP-OES).Effects of metal concentration, species, water media, and contact time were evaluated. Different concentrations (0.1, 1, 10 and 100 mg L−1) of Cd(II), Cr(VI), Pb(II), Ni(II) in aqueous solutions were prepared separately by dissolving their respective nitrate or potassium salt. These concentrations were selected based on a concentration range frequently observed in contaminated waters.29 To evaluate the removal efficiency under realistic environmental conditions, two aqueous test media (EPA soft water and marine water) were used, either with or without 0.5 mg L−1 of added Suwannee River Fulvic Acid (SRFA). The synthetic soft water and seawater solutions were prepared following the U.S. EPA protocol.30 NPs and metals were mixed together for different time periods of 1.5 h, 3 h, 6 h, 12 h, and 24 h, which were used to examine the effects of contact time on metal removal efficiency. In addition, effect of PVP–Fe3O4 NPs age was also evaluated. Freshly synthesized and three week old PVP–Fe3O4 NPs were used to remove metals (1 mg L−1) from soft water. The three weeks old NPs were sonicated 30 min before addition into metal solutions to help redisperse.
Metal adsorption per unit of adsorbent at time t was calculated by eqn (1).31
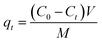 | (1) |
The removal percentage was calculated by eqn (2):
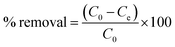 | (2) |
2.4 Modeling kinetics of adsorption
In order to investigate the mechanism and rate of the metal adsorption process, a pseudo-first-order equation32 was used to fit the data:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (3) |
In addition, a pseudo-second-order kinetic model was used, which is given by the following equation:33
| t/qt = 1/k2qe2 + t/qe (k0 = k2qe2) | (4) |
2.5 Statistical analysis
The reported data in this study are means of three replicates ± standard deviation (SD). One-way ANOVA was used to analyze the experiment variance (SPSS19.0 package, Chicago, IL) followed by the Tukey Test to determine statistical differences between treatments at “p < 0.05” level.3. Results and discussion
3.1 Characterization
Full information of the PVP–Fe3O4 characterization was given in the previous study.26 In brief, the median particle size and hydrodynamic size is 11.2 nm (interquartile range: 6.3–18.3 nm) and 127.4 ± 4.2 nm as measured by atomic force microscopy (AFM) and dynamic light scattering (DLS), respectively. The Fourier Transform infrared spectrometer (FTIR) result suggests that NPs are coated by PVP and likely through the PVP carbonyl group. In addition, thermogravimetric analysis (TGA) shows that 8.5% of mass of NPs belong to the PVP coating and 91.5% to the iron oxide cores. Based on X-ray diffraction (XRD), magnetite (Fe3O4) is the dominant phase of NPs.3.2 Metal removal in inorganic solutions
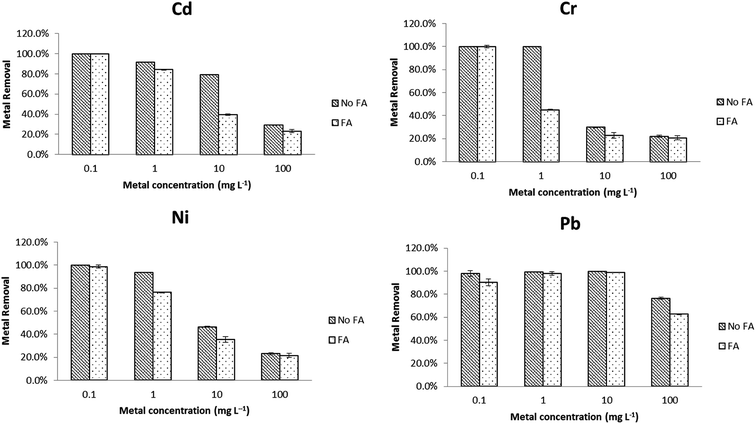
The metal removal efficiency using PVP–Fe3O4 NPs in EPA soft water (A) and EPA sea water (B) is shown in Fig. 1. In Fig. 1A (soft water), it is clear that at lowest metal concentration (0.1 mg L−1), removal was nearly complete for all four metals, while this was greater than 90% at 1 mg L−1 level of metals. At the highest concentrations (10 and 100 mg L−1), only Pb achieved an acceptable removal percentage (≥80%). In Fig. 1B (sea water), the removal percentages were above 90% for all four metal at 0.1 mg L−1 level, but at higher level (1 mg L−1) only Pb and Cr had more than 90% removal percentage. As with Fig. 1A, all metals when lower than 1 mg L−1 were reduced to acceptable concentrations, based on the Criterion Maximum Concentration (CMC) from US EPA. For Cd, Cr, Ni and Pb the CMC values are 18, 16, 470 and 65 μg L−1 in freshwater and 33, 1100, 74 and 210 μg L−1 in sea water.34 At the higher concentrations, only lead was reduced to an CMC ‘acceptable’ level, although addition of further nanoparticles would likely have improved removal. Typical metal concentration in freshwater and seawater are around or lower than CMC value and polluted systems can reach 0.38–12 mg L−1.8,35,36 Given this condition, PVP–Fe3O4 NPs could potentially control existing pollution effectively and be used to polish relatively clean waters in a cost effective manner. Addition of more NPs could make them suitable for remediation of more polluted waters, but the cost implications would need to be evaluated on a site-specific basis.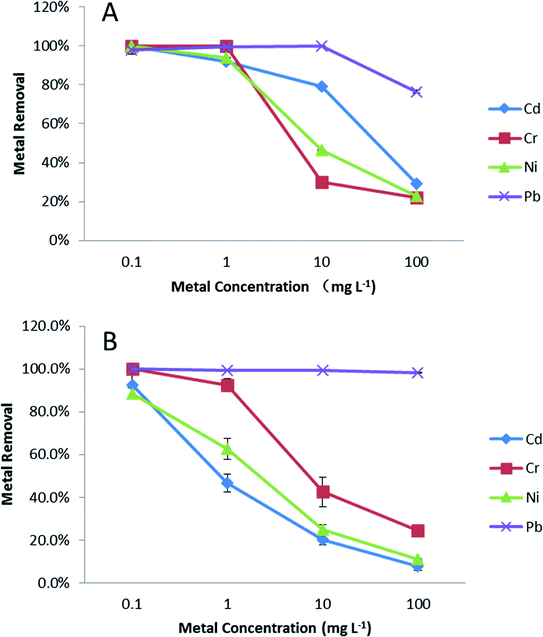 | ||
| Fig. 1 Effects of metal speciation on PVP–Fe3O4 NPs removal efficiency in different inorganic water media. (A) EPA soft water, (B) EPA sea water. Data are average of three replicates. | ||
In general, Fig. 1 shows that the removal percentages in soft water (Fig. 1A) were higher than sea water (Fig. 1B). The IEP for Fe3O4 NPs is at pH 6.5,37 although this value will depend on the nature of the NPs and these PVP coated particles show a non-pH dependent slightly positive charge.26 The different results for the two water types are most easily explained by charge shielding and neutralization leading to reduced electrostatic attraction between NPs and metals, although partly permeable NPs of this type show much more complex interactions with solutes and solvent.38 In the aqueous solution, metal ions can present as stable ions or hydrolyse to form a series of mononuclear and polynuclear hydroxyl as the following reaction39
| Mn+ + nH2O ↔ M(OH)n(m−n)− + nH+ |
| H2O + Fe–O− ↔ Fe–OH ↔ Fe–OH2+ |
Therefore, the adsorption of metal ions onto the surface of iron oxide nanoparticles is likely to be an electrostatic attraction between the positive metal ions and negatively charged surface of iron oxide.
It is clear that Pb has a higher adsorption to PVP–Fe3O4 NPs than other three metals. The binding is affected by differences in the metal's molecular mass, ion charges, ionic radius, hydration energy, and electrostatic and diffusional effects of the polymer coating.38,41 The ionic radius of Cr(VI), Ni(II), Cd(II) and Pb(II) are 58, 83, 109, and 133 pm, respectively.42 Pb(II) has the highest ionic radius, which resulted in higher binding power and higher adsorption. Other studies have shown that iron oxide NPs adsorb Pb much more strongly compared to other metals (e.g. Cu, Cd) in synthetic and natural water.43–45 In soft water (Fig. 1A), at higher concentrations (10 and 100 mg L−1), the metal removal followed the trend that Cr < Ni < Cd < Pb, which was same as their ionic radius sequence.
The effects of different metal initial concentrations on removal percentage are shown in Fig S1.† It was shown that, when initial concentration of metal was increased, removal efficiency decreased, as expected of an adsorption reaction. These results are similar as other studies15,46,47 and can be explained by the adsorption capacity and the chelating power of the ligand. At low metal concentration, there were more free and stronger binding sites on the surface of NPs, which caused higher metal removal efficiency. The maximum metal binding capacity of NPs limits their effectiveness at high metal concentration.
3.3 Effects on metal adsorption of the presence of Suwannee River fulvic acid
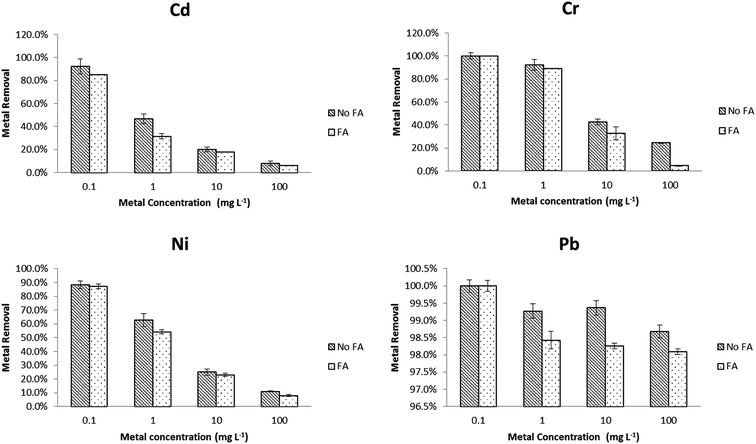
To better mimic real environmental conditions, metal removal tests in the same EPA water media were performed in the presence of 0.5 mg L−1 SRFA and compared with data from Section 1. The effects of adding SRFA to synthetic soft water and sea water are shown in Fig. 2 and 3, respectively. In soft water, there was no significant change (p < 0.05) for Pb with the addition of SRFA. For Cd, Cr and Ni, there was no significant change (p > 0.05) at 1 mg L−1 and 100 mg L−1 level. However, the addition of SRFA decreased the removal percentage from 80% to 40% and from 99% to 40% of the 10 mg L−1 Cd and 1 mg L−1 Cr, respectively. SRFA also significantly decreased Ni removal percentage more than 10% at 1, 10 mg L−1. At the lower metal concentrations, there was an excess of free surface sites of PVP–Fe3O4 NPs for metal binding; at the higher metal concentrations, the free binding sites of the NPs were saturated. Thus, SRFA could not affect metal binding at either very low (1 mg L−1) or very high (100 mg L−1) metal concentrations, only at the intermediate ratios were the magnetite and SRFA effectively in competition for metal interaction. SRFA had less effect in sea water compared with soft water. As shown in Fig. 3, there was no significant difference for Pb removal percentage after adding SRFA. However, binding significantly decreased 10% of Cd and Ni removal percentage at 1 mg L−1, and 20% of Cr removal percentage at 100 mg L−1. This simple picture of competition, is complicated by the possibility of SRFA aggregation in sea water48 and ternary interactions.49The adsorption capacities of PVP–Fe3O4 NPs at 10 mg L−1 metal level are shown in Table 1. In soft water without SRFA, the highest adsorption capacity of PVP–Fe3O4 NPs occurred for Pb followed by Cd, Ni and Cr, although Cd and Cr were comparable in the presence of SRFA. In seawater, the sequence was Pb, then Cr, with Ni and Cd comparable. In addition, in the presence of SRFA metal binding was reduced, in all cases and generally to a significant degree (p < 0.05). In general PVP–Fe3O4 had higher adsorption capacities in softer water than sea water, and adding SRFA significantly decreased adsorption under certain conditions. A comparison from the recent literature shows that the magnetite NPs used here in soft water had higher Pb binding capacities: 59.6–61.6 mg g−1 than magnetite nanospheres: 18.47 mg g−1,29 Fe3O4/SiO2 nanocomposites: 17.65 mg g−1,50 MnO nanocomposites: 21 mg g−1,51 and magnetic ion-imprinted polymer NPs: 48.1 mg g−1.52 Also, our NPs had higher adsorption capacities: 17.9–25.5 mg g−1 for Cr than other materials, such as magnetite nanospheres: 8.9 mg g−1,29 amino-modified Fe3O4 NPs: 11.2 mg g−1,53 and ceria hollow nanospheres: 15.4 mg g−1.54 In addition, the adsorption capacities (15.01–29.86 mg g−1) for Ni were higher than Fe3O4 NPs: 11.53 mg g−1.55 In general, these NPs were highly effective for Pb removal from the aqueous phase and sufficiently so for the other metals tested.
| Soft water | Sea water | |||
|---|---|---|---|---|
| Without SRFA | With SRFA | Without SRFA | With SRFA | |
| Cd | 43.92 ± 2.56 | 23.66 ± 2.32 | 12.08 ± 0.55 | 11.62 ± 1.03 |
| Cr | 17.98 ± 0.86 | 13.87 ± 0.98 | 25.52 ± 1.02 | 22.89 ± 1.78 |
| Ni | 29.86 ± 1.45 | 21.23 ± 0.97 | 15.01 ± 1.39 | 13.47 ± 0.65 |
| Pb | 61.67 ± 4.57 | 55.33 ± 3.41 | 59.62 ± 3.07 | 20.19 ± 1.49 |
The reduced metal binding capacity in the presence of SRFA could be explained by the following different mechanisms: (1) SRFA could cause aggregation of PVP–Fe3O4 NPs, reducing specific surface area and affecting adsorption capacity. Earlier studies showed no aggregation using PVP as a capping agent.24 However, at these higher NP concentrations, increased aggregation has been observed.56 (2) SRFA might have modified the surface structure of the Fe3O4 NPs causing changes in crystallinity and degradation of the NPs as we have seen with PVP-coated ceria NPs.57 The changed crystal structure could have resulted in reduced magnetic separation. (3) The SRFA could have act as a competitive phase for either PVP–Fe3O4 NPs or metal ions which could affect adsorption capacity negatively.58 Such well known competition (between metal ions and other ions in the sea water) also could explain why the adsorption capacities were lower in sea water than soft water for Cd, Ni and Pb. However, adsorption capacity on Cr is higher in sea water than softer water, possibly related to the dominance of the anion CrO42− (Visual MINTEQ 3.1; Tables S2–S5†).
3.4 Kinetic studies
Binding kinetics is important when considering water treatment applications; rapid binding reduces treatment time and cost, while longer contact times may allow greater removal. Fig. 4 shows effects of contact time on metal adsorption over 24 hours, using initial metal concentration at 1 mg L−1 in soft water (Fig. 4A) and sea water (Fig. 4B) as examples. In soft water (Fig. 4A), Pb binding was immediate and complete (100% bound at earliest time). For Cr and Ni, ≥75% of all metal was adsorbed within 1.5 hours, with increases over 24 hours, becoming complete after 24 hours (84.0% to 100%, and 70.8% to 92.6%, respectively). Between 1.5 and 24 hours Cd binding significantly increased from 30.3% to 93.5%. In sea water (Fig. 4B), there was no difference for Pb. However, Cr removal percentage significantly increased from 77.2% to 98.8% during 24 h. Cd and Ni had less than 10% removal percentage increase from 1.5 h to 24 h. It is obvious that in sea water, Cd and Ni had lower removal percentage and slower adsorption process compared with soft water. This might be caused by salt in the sea water, which could compete with metal ions binding with NPs.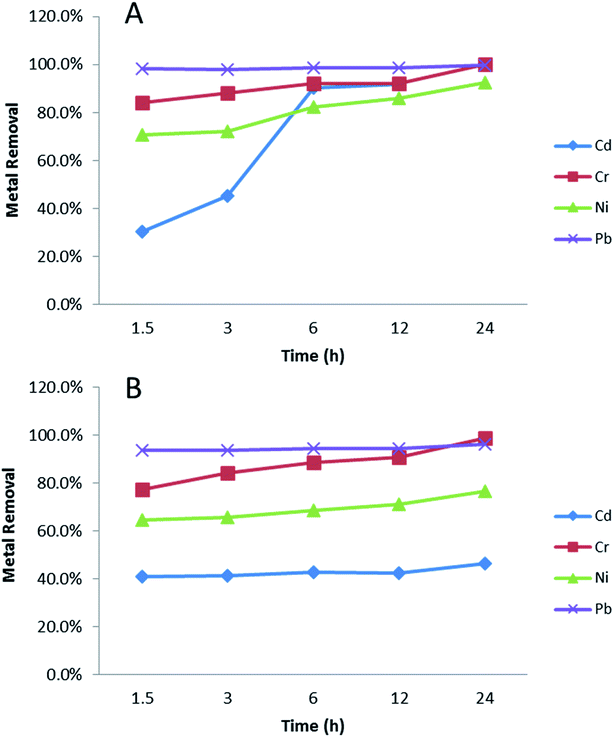 | ||
| Fig. 4 The influence of contact time in metal (1 mg L−1) adsorption. (A) EPA soft water, (B) EPA sea water. Data are average of three replicates. | ||
PVP–Fe3O4 NPs removed nearly 100% Pb, and most (>60%) of Cr and Ni in the first 1.5 h of mixing. This measured reaction time is shorter than some other studies, such as 2.5 hours for activated carbon59 and 4 hours for clay minerals.60 However, it was longer than for some other materials, such PMDA/TMSPEDA hybrid polymeric nano-composite61 and graphene oxide-based microbots.62 Nevertheless, the low cost, simplicity and low environmental footprint of the magnetite NPs27,28 may mean they are economically viable.
Adsorption kinetic models allow the estimation of adsorption rate and provide insights into rate expression characteristics of possible reaction mechanisms.63 Fig. 5 shows the results of a pseudo-second-order adsorption kinetic adsorption model for the four metals in two media, showing similarities due to the fact that most adsorption occurs prior to the 1.5 hour time point. This time point was chosen as the first due to logistical/time constraints in the experiments. Cd adsorption in seawater was the only one which showed a difference, due to the low initial adsorption followed by rapid adsorption between in its major species in soft water was Cd(CO3)22− and in sea water were CdCl+ and CdCl2 (aq) (Table S2†). Cd(CO3)22− has negative charge, so it could bind with PVP–Fe3O4 NPs easily. However, in Fig. 4B there was almost no changes over time for Cd. The possible reason might be that the adsorption occurred at the very beginning and was done before the measurement starts (1.5 h). This assumption was also supported by Fig. 5B, where Cd had a different trend with other three metals. Also, major species of Cr in both water media was CrO42− (Table S3†), which might explain why Cr adsorption had similar trend in both water media. It seems metal speciation could affect adsorption kinetic.
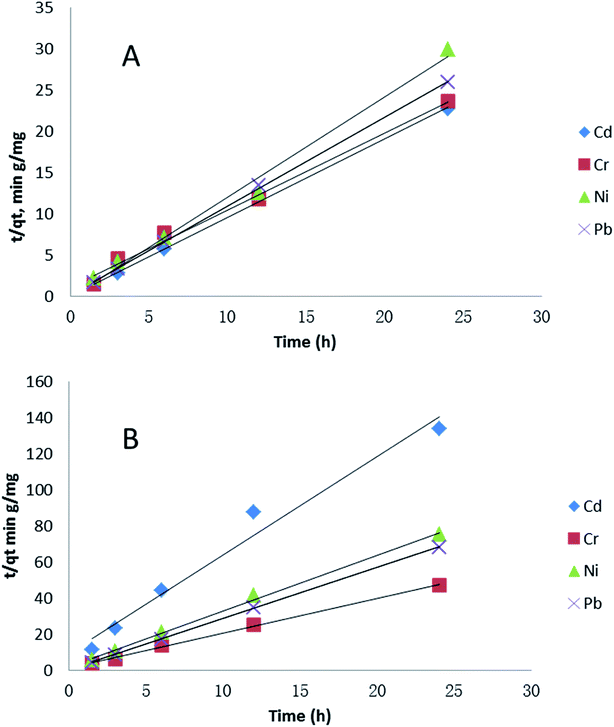 | ||
| Fig. 5 Effect of contact time on pseudo-second-order kinetics of four metals adsorption (1 mg L−1 initial metal concentration) in different water media: (A) softer water and (B) sea water. | ||
The values of correlation factor R2, obtained from the plots of pseudo-second-order kinetics shown in Table S1† are greater (R2 > 0.99) than that of the pseudo-first-order. This suggests that adsorption of Cd, Cr, Ni and Pb onto PVP–Fe3O4 NPs follows a pseudo-second-order binding. The pseudo second-order adsorption model is based on the assumption that the rate-controlling step of adsorption involved covalent bond through sharing or exchange of electrons between adsorbent and adsorbate,64 which means the rate depends on concentrations of PVP–Fe3O4 NPs and the relevant metal. The pseudo-second-order has also been reported for some heavy metals on many adsorbents such as functionalized magnetic mesoporous silica,65 polyethylenimine grafted magnetic porous adsorbent,66 monodispersed magnetite NPs.15
3.5 Different storage period of NPs
Practical use of these NPs for treatment, likely requires their production and storage for later use. In order to quantify the effects of ageing, freshly synthesized PVP–Fe3O4 NPs which had been aged for 3 weeks at room temperature. Results are shown in Fig. 6 for 1 mg L−1 in soft water. There was no difference between these two NPs (fresh and stored) in terms of Pb removal efficiency, again showing excellent removal after the storage period. However, there was a large and significant reduction in the NPs ability to remove Cr. The removal percentage is above 80–100% for fresh NPs but only 20–40% for aged ones. In the case of Cd, removal percentages were identical after 6 hours for both the fresh and stored NPs. However, the stored NPs had a reduced capacity at shorter time periods. Possible reason for these differences include agglomeration of NPs leading to reduced specific surface area, or microbial growth leading to several potential alterations. Dilution from stock solutions could also explain the Cd removal before 6 hours, since agglomeration for these NPs is concentration dependent the concentration range.56 Dilution into the test solution likely promotes dispersion but with a time delay. In future work, with the exception of Pb, freshly synthesized NPs should be used, or storage conditions investigated further e.g. NPs should be diluted prior to use, stored at low temperature.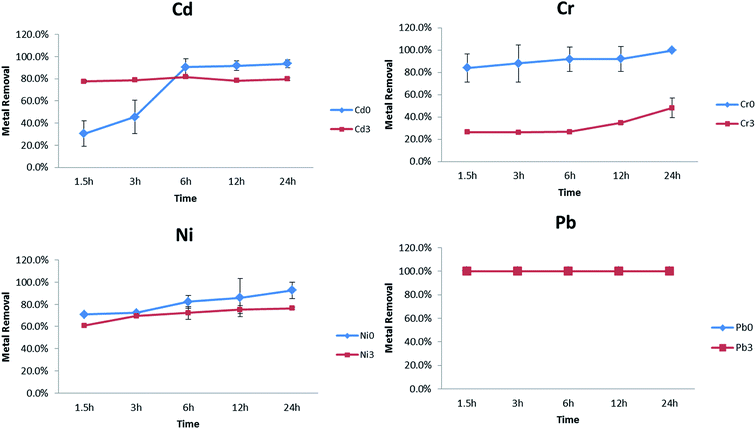 | ||
| Fig. 6 Comparison between fresh and aged (3 weeks old) synthesized nanoparticles' efficiency to remove metals from soft water. Metal concentrations were 1 mg L−1. | ||
4. Conclusion
In this study, we used PVP–Fe3O4 NPs for metal remediation in different water media and time, designed to mimic real environmental conditions. Results showed these NPs could reduce metals efficiently in both soft water and seawater. Especially for Pb, the adsorption could be done in less than 1.5 hour and the removal percentage could achieve 100%. The equilibrium data were fitted well by the pseudo-second-order kinetic model. In addition, PVP–Fe3O4 NPs are synthesized through a facile, environmental friendly and cost-effective hydrothermal technique. In previous study, it has been proved that PVP–Fe3O4 NPs could remove oil efficiently under different environmental conditions. Therefore, it is expected that PVP–Fe3O4 NPs have wide applicability in the water remediation. Further investigation is required related to storage conditions in the feasibility of using the NPs in a commercial setting.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We would like to thank the Center for Environmental Nanoscience & Risk and University of South Carolina for financial support. And the funding from Office of Science and Technology of Jinzhong, China (Y182012).References
- A. James, Heavy metals in fish and shellfish, EOS, 2012, 20, 07–12 Search PubMed.
- R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat and A. Alsaedi, Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions, Chem. Eng. J., 2015, 260, 469–477 CrossRef CAS.
- Y. Finkelstein, M. E. Markowitz and J. F. Rosen, Low-level lead-induced neurotoxicity in children: an update on central nervous system effects, Brain Res. Rev., 1998, 27, 168–176 CrossRef CAS PubMed.
- A. Z. Pollack, E. F. Schisterman, L. R. Goldman, S. L. Mumford, P. S. Albert, R. L. Jones and J. Wactawski-Wende, Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women, Environ. Health Perspect., 2011, 119, 1156 CrossRef CAS PubMed.
- P. Krauter, B. Daily Jr, V. Dibley, H. Pinkart and T. Legler, Perchlorate and nitrate remediation efficiency and microbial diversity in a containerized wetland bioreactor, Int. J. Phytorem., 2005, 7, 113–128 CrossRef CAS PubMed.
- M. Adrees, S. Ali, M. Rizwan, M. Ibrahim, F. Abbas, M. Farid, M. Zia-ur-Rehman, M. K. Irshad and S. A. Bharwana, The effect of excess copper on growth and physiology of important food crops: a review, Environ. Sci. Pollut. Res., 2015, 22, 8148–8162 CrossRef CAS PubMed.
- USEPA, https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants, accessed 2.1, 2017.
- J. M. Allen, A. A. Cuthbertson, H. K. Liberatore, S. Y. Kimura, A. Mantha, M. A. Edwards and S. D. Richardson, Showering in Flint, MI: Is there a DBP problem?, J. Environ. Sci., 2017, 58, 271–284 CrossRef PubMed.
- J. Hong, Y. Wang, S. McDermott, B. Cai, C. M. Aelion and J. Lead, The use of a physiologically-based extraction test to assess relationships between bioaccessible metals in urban soil and neurodevelopmental conditions in children, Environ. Pollut., 2016, 212, 9–17 CrossRef CAS PubMed.
- USEPA, Hazard Standards for Lead in Paint, Dust and Soil, 2001, accessed 2017.2.24 Search PubMed.
- K. Panchal and R. Subramanian, A journey from heavy metal to metallothionein: a critical review of heavy metal remediation by plants, Int. J. Sci. Res., 2016, 5, 5 Search PubMed.
- E. Pilon-Smits, Phytoremediation, Annu. Rev. Plant Biol., 2005, 56, 15–39 CrossRef CAS PubMed.
- S. S. Patil, U. U. Shedbalkar, A. Truskewycz, B. A. Chopade and A. S. Ball, Nanoparticles for environmental clean-up: a review of potential risks and emerging solutions, Environmental Technology & Innovation, 2016, 5, 10–21 Search PubMed.
- K. L. Garner and A. A. Keller, Emerging patterns for engineered nanomaterials in the environment: a review of fate and toxicity studies, J. Nanopart. Res., 2014, 16, 2503 CrossRef.
- Y. Bagbi, A. Sarswat, D. Mohan, A. Pandey and P. R. Solanki, Lead (Pb2+) adsorption by monodispersed magnetite nanoparticles: surface analysis and effects of solution chemistry, J. Environ. Chem. Eng., 2016, 4, 4237–4247 CrossRef CAS.
- S. Palchoudhury and J. R. Lead, A Facile and Cost-Effective Method for Separation of Oil–Water Mixtures Using Polymer-Coated Iron Oxide Nanoparticles, Environ. Sci. Technol., 2014, 48, 14558–14563 CrossRef CAS PubMed.
- J.-F. Liu, Z.-s. Zhao and G.-b. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS PubMed.
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I.-C. Hwang and K. S. Kim, Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal, ACS Nano, 2010, 4, 3979–3986 CrossRef CAS PubMed.
- C. Shan, Z. Ma, M. Tong and J. Ni, Removal of Hg(II) by poly (1-vinylimidazole)-grafted Fe3O4@ SiO2 magnetic nanoparticles, Water Res., 2015, 69, 252–260 CrossRef CAS PubMed.
- F. M. Koehler, M. Rossier, M. Waelle, E. K. Athanassiou, L. K. Limbach, R. N. Grass, D. Günther and W. J. Stark, Magnetic EDTA: coupling heavy metal chelators to metal nanomagnets for rapid removal of cadmium, lead and copper from contaminated water, Chem. Commun., 2009, 4862–4864 RSC.
- D. Zhang, S. Wei, C. Kaila, X. Su, J. Wu, A. B. Karki, D. P. Young and Z. Guo, Carbon-stabilized iron nanoparticles for environmental remediation, Nanoscale, 2010, 2, 917–919 RSC.
- H. Wang, Y.-F. Yu, Q.-W. Chen and K. Cheng, Carboxyl-functionalized nanoparticles with magnetic core and mesopore carbon shell as adsorbents for the removal of heavy metal ions from aqueous solution, Dalton Trans., 2011, 40, 559–563 RSC.
- M. Tejamaya, I. Römer, R. C. Merrifield and J. R. Lead, Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media, Environ. Sci. Technol., 2012, 46, 7011–7017 CrossRef CAS PubMed.
- A. Hitchman, G. H. Smith, Y. Ju-Nam, M. Sterling and J. R. Lead, The effect of environmentally relevant conditions on PVP stabilised gold nanoparticles, Chemosphere, 2013, 90, 410–416 CrossRef CAS PubMed.
- T. Stoiber, M.-N. Croteau, I. Römer, M. Tejamaya, J. R. Lead and S. N. Luoma, Influence of hardness on the bioavailability of silver to a freshwater snail after waterborne exposure to silver nitrate and silver nanoparticles, Nanotoxicology, 2015, 9, 918–927 CrossRef PubMed.
- S. Mirshahghassemi and J. R. Lead, Oil Recovery from Water under Environmentally Relevant Conditions Using Magnetic Nanoparticles, Environ. Sci. Technol., 2015, 49, 11729–11736 CrossRef CAS PubMed.
- S. Mirshahghassemi, B. Cai and J. R. Lead, Evaluation of polymer-coated magnetic nanoparticles for oil separation under environmentally relevant conditions: effect of ionic strength and natural organic macromolecules, Environ. Sci.: Nano, 2016, 3, 780–787 RSC.
- S. Mirshahghassemi, A. D. Ebner, B. Cai and J. R. Lead, Application of high gradient magnetic separation for oil remediation using polymer-coated magnetic nanoparticles, Sep. Purif. Technol., 2017, 179, 328–334 CrossRef CAS.
- M. Kumari, C. U. Pittman and D. Mohan, Heavy metals [chromium(VI) and lead(II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres, J. Colloid Interface Sci., 2015, 442, 120–132 CrossRef CAS PubMed.
- C. I. Weber, Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency, 1991 Search PubMed.
- M. Nameni, M. A. Moghadam and M. Arami, Adsorption of hexavalent chromium from aqueous solutions by wheat bran, Int. J. Environ. Sci. Technol., 2008, 5, 161–168 CrossRef CAS.
- S. Largegren, About the theory of so-called adsorption of soluble substances, K. Sven. Vetenskapsakad. Handl., 1898, 241, 1–39 Search PubMed.
- J.-P. Simonin, On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics, Chem. Eng. J., 2016, 300, 254–263 CrossRef CAS.
- USEPA, https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table,https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table, accessed 11.14, 2018.
- Water Quality Assessment and Correlation Study of Physico-Chemical Parameters of Sukinda Chromite Mining Area, Odisha, India, in Environmental Pollution, ed. V. P. Singh, S. Yadav, and R. N. Yadava, Springer Singapore, Singapore, 2018, pp. 357–370 Search PubMed.
- S. Grellier, P. Seyler, C. Petitjean, M.-P. Bonnet, W. Thothong and J.-L. Janeau, in Socio-Ecological Dimensions of Infectious Diseases in Southeast Asia, ed. S. Morand, J.-P. Dujardin, R. Lefait-Robin and C. Apiwathnasorn, Springer Singapore, Singapore, 2015, pp. 57–74, DOI:10.1007/978-981-287-527-3_5.
- L. Zhu, D. Pan, L. Ding, F. Tang, Q. Zhang, Q. Liu and S. Yao, Mixed hemimicelles SPE based on CTAB-coated Fe3O4/SiO2 NPs for the determination of herbal bioactive constituents from biological samples, Talanta, 2010, 80, 1873–1880 CrossRef CAS PubMed.
- H. P. Van Leeuwen, J. F. Duval, J. P. Pinheiro, R. Blust and R. M. Town, Chemodynamics and bioavailability of metal ion complexes with nanoparticles in aqueous media, Environ. Sci.: Nano, 2017, 4, 2108–2133 RSC.
- T. M. Petrova, L. Fachikov and J. Hristov, The magnetite as adsorbent for some hazardous species from aqueous solutions: a review, arXiv preprint arXiv:1104.5647, 2011.
- N. N. Nassar, Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents, J. Hazard. Mater., 2010, 184, 538–546 CrossRef CAS PubMed.
- J. r. m. F. Duval, R. M. Town and H. P. Van Leeuwen, Lability of nanoparticulate metal complexes at a macroscopic metal responsive (bio) interface: expression and asymptotic scaling laws, J. Phys. Chem. C, 2018, 122, 6052–6065 CrossRef CAS.
- C. Quintelas, Z. Rocha, B. Silva, B. Fonseca, H. Figueiredo and T. Tavares, Removal of Cd(II), Cr(VI), Fe(III) and Ni(II) from aqueous solutions by an E. coli biofilm supported on kaolin, Chem. Eng. J., 2009, 149, 319–324 CrossRef CAS.
- C. Zhang, Z. Yu, G. Zeng, B. Huang, H. Dong, J. Huang, Z. Yang, J. Wei, L. Hu and Q. Zhang, Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd, Chem. Eng. J., 2016, 284, 247–259 CrossRef CAS.
- C. Tamez, R. Hernandez and J. Parsons, Removal of Cu(II) and Pb(II) from aqueous solution using engineered iron oxide nanoparticles, Microchem. J., 2016, 125, 97–104 CrossRef CAS PubMed.
- J. E. Groenenberg and S. Lofts, The use of assemblage models to describe trace element partitioning, speciation, and fate: a review, Environ. Toxicol. Chem., 2014, 33, 2181–2196 CrossRef CAS PubMed.
- E. I. Anastasova, V. Ivanovski, A. F. Fakhardo, A. I. Lepeshkin, S. Omar, A. S. Drozdov and V. V. Vinogradov, A pure magnetite hydrogel: synthesis, properties and possible applications, Soft Matter, 2017, 13, 8651–8660 RSC.
- M. Jain, M. Yadav, T. Kohout, M. Lahtinen, V. K. Garg and M. Sillanpää, Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution, Water Resources and Industry, 2018, 20, 54–74 CrossRef.
- J. R. Lead, K. J. Wilkinson, K. Starchev, S. Canonica and J. Buffle, Determination of diffusion coefficients of humic substances by fluorescence correlation spectroscopy: role of solution conditions, Environ. Sci. Technol., 2000, 34, 1365–1369 CrossRef CAS.
- N. N. Seda, F. Koenigsmark and T. M. Vadas, Sorption and coprecipitation of copper to ferrihydrite and humic acid organomineral complexes and controls on copper availability, Chemosphere, 2016, 147, 272–278 CrossRef CAS PubMed.
- M. Mahdavi, M. B. Ahmad, M. J. Haron, Y. Gharayebi, K. Shameli and B. Nadi, Fabrication and characterization of SiO2/(3-aminopropyl) triethoxysilane-coated magnetite nanoparticles for lead(II) removal from aqueous solution, J. Inorg. Organomet. Polym. Mater., 2013, 23, 599–607 CrossRef CAS.
- S. Rajput, C. U. Pittman and D. Mohan, Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water, J. Colloid Interface Sci., 2016, 468, 334–346 CrossRef CAS PubMed.
- M. Fayazi, M. A. Taher, D. Afzali, A. Mostafavi and M. Ghanei-Motlagh, Synthesis and application of novel ion-imprinted polymer coated magnetic multi-walled carbon nanotubes for selective solid phase extraction of lead(II) ions, Mater. Sci. Eng., C, 2016, 60, 365–373 CrossRef CAS PubMed.
- S.-H. Huang and D.-H. Chen, Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent, J. Hazard. Mater., 2009, 163, 174–179 CrossRef CAS PubMed.
- C.-Y. Cao, Z.-M. Cui, C.-Q. Chen, W.-G. Song and W. Cai, Ceria hollow nanospheres produced by a template-free microwave-assisted hydrothermal method for heavy metal ion removal and catalysis, J. Phys. Chem. C, 2010, 114, 9865–9870 CrossRef CAS.
- Y. Sharma and V. Srivastava, Separation of Ni(II) ions from aqueous solutions by magnetic nanoparticles, J. Chem. Eng. Data, 2009, 55, 1441–1442 CrossRef.
- A. Alabresm, Y. P. Chen, A. W. Decho and J. Lead, A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria, Sci. Total Environ., 2018, 630, 1292–1297 CrossRef CAS PubMed.
- R. C. Merrifield, K. P. Arkill, R. E. Palmer and J. R. Lead, A High Resolution Study of Dynamic Changes of Ce2O3 and CeO2 Nanoparticles in Complex Environmental Media, Environ. Sci. Technol., 2017, 51, 8010–8016 CrossRef CAS PubMed.
- B. L. Lau, W. C. Hockaday, K. Ikuma, O. Furman and A. W. Decho, A preliminary assessment of the interactions between the capping agents of silver nanoparticles and environmental organics, Colloids Surf., A, 2013, 435, 22–27 CrossRef CAS.
- L. Wang, L. Yang, Y. Li, Y. Zhang, X. Ma and Z. Ye, Study on adsorption mechanism of Pb(II) and Cu(II) in aqueous solution using PS–EDTA resin, Chem. Eng. J., 2010, 163, 364–372 CrossRef CAS.
- D. Ozdes, C. Duran and H. B. Senturk, Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay, J. Environ. Manage., 2011, 92, 3082–3090 CrossRef CAS PubMed.
- I. H. Alsohaimi, S. M. Wabaidur, M. Kumar, M. A. Khan, Z. A. Alothman and M. A. Abdalla, Synthesis, characterization of PMDA/TMSPEDA hybrid nano-composite and its applications as an adsorbent for the removal of bivalent heavy metals ions, Chem. Eng. J., 2015, 270, 9–21 CrossRef CAS.
- D. Vilela, J. Parmar, Y. Zeng, Y. Zhao and S. Sánchez, Graphene-based microbots for toxic heavy metal removal and recovery from water, Nano Lett., 2016, 16, 2860–2866 CrossRef CAS PubMed.
- A. Iriel, S. P. Bruneel, N. Schenone and A. F. Cirelli, The removal of fluoride from aqueous solution by a lateritic soil adsorption: Kinetic and equilibrium studies, Ecotoxicol. Environ. Saf., 2018, 149, 166–172 CrossRef CAS PubMed.
- A. Murugesan, L. Ravikumar, V. SathyaSelvaBala, P. SenthilKumar, T. Vidhyadevi, S. D. Kirupha, S. Kalaivani, S. Krithiga and S. Sivanesan, Removal of Pb(II), Cu(II) and Cd(II) ions from aqueous solution using polyazomethineamides: Equilibrium and kinetic approach, Desalination, 2011, 271, 199–208 CrossRef CAS.
- G. Li, Z. Zhao, J. Liu and G. Jiang, Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica, J. Hazard. Mater., 2011, 192, 277–283 CAS.
- Y. Pang, G. Zeng, L. Tang, Y. Zhang, Y. Liu, X. Lei, Z. Li, J. Zhang and G. Xie, PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions, Desalination, 2011, 281, 278–284 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10104g |
| This journal is © The Royal Society of Chemistry 2020 |