Open Access Article
Open Access ArticleSynthesis and characterization of carboxylated PBAMO copolymers as promising prepolymers for energetic binders
Minkyung Lim†
a,
Meilan Bu†a,
Yoorim Janga,
Jongoh Jeongb,
Sitae Noh*c and
Hakjune Rhee *ad
*ad
aDepartment of Bionanotechnology, Hanyang University, 55 Hanyangdaehak-ro, Sangnok-gu, Ansan, Gyeonggi-do 15588, South Korea. E-mail: hrhee@hanyang.ac.kr
bNOROO Paint & Coatings Co., Ltd., 351, Bakdal-ro, Manan-gu, Anyang-si, Gyeonggido 13977, South Korea
cDepartment of Materials Science and Chemical Engineering, Hanyang University, 55 Hanyangdaehak-ro, Sangnok-gu, Ansan, Gyeonggi-do 15588, South Korea
dDepartment of Applied Chemistry, Hanyang University, 55 Hanyangdaehak-ro, Sangnok-gu, Ansan, Gyeonggi-do 15588, South Korea
First published on 2nd March 2020
Abstract
The carboxylated poly[3,3-bis(3-azidomethyl)oxetane] (PBAMO) copolymers (poly(BAMO-carboxylate)) were synthesized by substitution of poly[3,3-bis(3-chloromethyl)oxetane] (PBCMO) with potassium carboxylate and sodium azide in DMSO. The synthesized compounds were characterized using various analytical techniques, such as Fourier-transform infrared (FT-IR) spectroscopy, inverse-gated decoupling 13C-nuclear magnetic resonance (13C NMR) spectroscopy, gel permeation chromatography (GPC), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), calorimetry, friction, and impact sensitivity analysis. These poly(BAMO-carboxylate) compounds have better thermal properties, with lower glass transition temperatures (ranging from −43 °C to −51 °C) than PBAMO (−37 °C) and higher thermal decomposition temperatures (233–237 °C) than PBAMO (211 °C). Moreover, poly(BAMO0.80-octanoate0.20) and poly(BAMO0.78-decanoate0.22) have higher heats of combustion (5226 and 5665 kJ mol−1, respectively) and negative formation enthalpies (−0.17 and −0.55 kJ g−1, respectively), while PBAMO has lower heat of combustion (3125 kJ mol−1) and positive formation enthalpy (0.06 kJ g−1). The poly(BAMO-carboxylate) compounds have higher values (38–50 J) than that of PBAMO (14 J) in the impact sensitivities. This is a valuable study for improving the properties of PBAMO, which is a high energetic polymeric binder but difficult to handle because of its sensitivity. Therefore, poly(BAMO-carboxylate) could be a good candidate as a prepolymer for designing the energetic polymeric binder.
1. Introduction
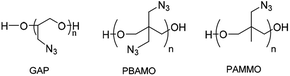
Solid rocket propellants are mixtures consisting of polymeric binders, high-energy additives, curing agents, oxidizers, metallic fuel additives, burning rate modifiers, etc.1–5 Among these components, the polymeric binder plays an important role in determining structural integrity, which in turn affects the performance of the propellant.6–8 The binders are typically cross-linked polymers, and hydroxyl-terminated poly(butadiene) (HTPB) is the most widely used prepolymer. The HTPB propellant has high specific impulse with the ammonium perchlorate (AP) oxidizer. However, the HTPB/AP binder releases HCl gas, which causes environmental pollution due to the propellant plumes.4,9 Recent propellant research has been aimed at developing high-energy binders that can provide better performance and improve the overall energy level to replace HTPB. Azide-based (–N3) polymers like glycidyl azide polymer (GAP), poly[3,3-bis(3-azidomethyl)oxetane] (PBAMO), and poly[(3-azidomethyl)methyloxetane] (PAMMO) are attracting attention, because they release high energy when decomposed (Scheme 1).10–12Among these polymers, GAP has a high positive enthalpy of formation (+957 kJ kg−1), a low glass transition temperature (Tg = −49 °C), low sensitivity, and good compatibility with high-energy oxidizers like ammonium dinitramide (ADN) and hydrazinium nitroformate (HNF).1,13–16 Furthermore, GAP produces chlorine-free propellant plumes with ammonium nitrate (AN) instead of AP, thus curbing environmental pollution. However, the bulky and polar azide groups in GAP induce low flexibility of backbone due to hindrance of motion in molecular chain, leading to drawbacks such as poor low-temperature characteristics.17–19 To improve the thermal properties of GAP, GAP copolymers such as fluorinated GAP copolymer,20 GAP-THF copolymer,21 GAP-PEG copolymer,22 and GAP-PBAMO copolymer18,23,24 have been synthesized. These GAP copolymers have better thermal properties than GAP.
Previously, we developed carboxylated GAP copolymers as prepolymers for designing high-energy binders.25 We focused on improving the thermal properties of GAP copolymer by the introduction of long-chain carboxylates like butyrate, octanoate, 2-ethyl hexanoate, isonanoate, and decanoate.
The synthesized carboxylated GAP copolymers have better thermal properties owing to their lower glass transition temperatures (ranging from −48 °C to −55 °C) than GAP (−49 °C) and thermal decomposition temperatures (228–230 °C) similar to that of GAP (227 °C). Moreover, carboxylated GAP copolymers have higher heats of combustion and negative formation enthalpies compared to GAP.
As an extension study of the synthesis of GAP copolymers, carboxylated PBAMO copolymers were synthesized and characterized herein. PBAMO containing two azide bonds has higher energy, but the glass transition temperature (Tg) and crystallinity of PBAMO are relatively high due to its symmetric structure.26,27 Because of these properties, practicality of PBAMO is limited;28 therefore, the development of new PBAMO copolymers is of importance. Based on the carboxylated GAP copolymer study, PBAMO copolymer was substituted with 20% long-chain carboxylates by selecting butanoate, octanoate, and decanoate. The resultant carboxylated PBAMO copolymers have lower glass transition temperatures, higher thermal decomposition temperatures, and lower sensitivities than PBAMO. Considering its excellent performance, the carboxylated PBAMO copolymer is a promising prepolymer candidate for energetic binders.
2. Results and discussion
2.1 Synthesis and characterization of poly(BAMO-carboxylate) compounds
Some GAP copolymers substituted with long-chain carboxylates (butyrate, octanoate, 2-ethyl hexanoate, isononanoate, and decanoate) have been reported previously.25 Poly(GA-carboxylate) compounds with three different azide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carboxylate molar ratios (90
carboxylate molar ratios (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and 70
20, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) were synthesized and characterized. Most of these poly(GA-carboxylate) compounds have properties similar to each other and exhibited better properties than GAP. Therefore, we selected butanoate, octanoate, and decanoate to synthesize PBAMO copolymers substituted with 20 mol% carboxylate.
30) were synthesized and characterized. Most of these poly(GA-carboxylate) compounds have properties similar to each other and exhibited better properties than GAP. Therefore, we selected butanoate, octanoate, and decanoate to synthesize PBAMO copolymers substituted with 20 mol% carboxylate.
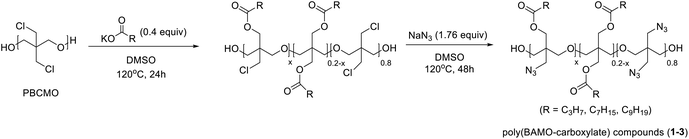
PBAMO is generally synthesized by using sodium azide with poly[3,3-bis(3-chloromethyl)oxetane] (PBCMO). First, we synthesized poly(BAMO-carboxylate) compounds by sequential addition of sodium azide and potassium carboxylate to PBCMO. However, due to the steric congestion in PBAMO containing two azide groups on one carbon, the substitution of the residual C–Cl bond with carboxylate was not completed at 120 °C. Thus, poly(BAMO-carboxylate) compounds were synthesized by the first substitution of PBCMO with relatively bulky carboxylate, followed by addition of azide group. Substitution of all C–Cl bonds was completed after 48 h using 1.76 equivalents of sodium azide at 120 °C. The synthesized poly(BAMO-carboxylate) compounds were analyzed by FT-IR spectroscopy, inverse-gated decoupling 13C-NMR spectroscopy, and GPC to confirm successful synthesis (Table 1).
| Entry | Compositiona | [Potassium carboxylate]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [sodium azide] mol% [sodium azide] mol% |
R | Yield (%) | Mwb (g mol−1) | Mnb (g mol−1) | PDIb | Tgc (°C) | Tdecd (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated by inverse-gated decoupling 13C NMR spectroscopy.b Measured by GPC.c Measured by DSC.d Measured by TGA (reported 1st Tdec). | |||||||||
| 1 | PBCMO | — | — | — | 2250 | 1850 | 1.22 | — | — |
| 2 | PBAMO | — | — | — | 2460 | 2030 | 1.21 | −37 | 211 |
| 3 | Poly(BAMO0.79-butyrate0.21) (1) | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
C3H7 | 92 | 2720 | 2080 | 1.30 | −43 | 233 |
| 4 | Poly(BAMO0.80-octanoate0.20) (2) | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
C7H15 | 90 | 2910 | 2390 | 1.22 | −47 | 237 |
| 5 | Poly(BAMO0.78-decanoate0.22) (3) | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
C9H19 | 91 | 2980 | 2440 | 1.22 | −51 | 237 |
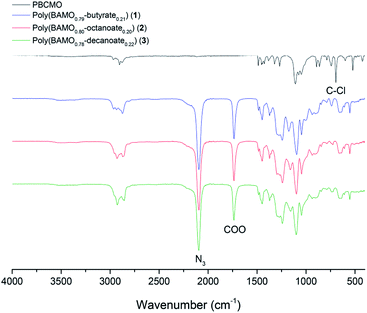
The completion of the reaction was confirmed by FT-IR spectroscopy (Fig. 1). Compared with the starting material PBCMO, the azide N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band at 2091 cm−1 and ester C
N stretching band at 2091 cm−1 and ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1735 cm−1 increased in intensity, while the C–Cl stretching band at 700 cm−1 disappeared as the reactions proceeded to completion.
O stretching band at 1735 cm−1 increased in intensity, while the C–Cl stretching band at 700 cm−1 disappeared as the reactions proceeded to completion.
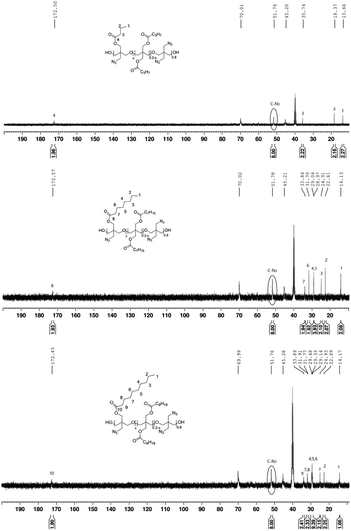
The composition of the polymer was analyzed by a quantitative 13C NMR spectroscopic study based on inverse-gated decoupling.25,29,30 The structures and successful synthesis of poly(BAMO-carboxylate) (1–3) were confirmed from the 13C NMR spectra. The compositions of poly(BAMO0.79-butyrate0.21) (1), poly(BAMO0.80-octanoate0.20) (2), and poly(BAMO0.78-decanoate0.22) (3) were calculated by averaging the integral values of each peak in the inverse-gated decoupling 13C NMR spectra (Fig. 2). The substitution ratio of azide and carboxylate in each compound was almost similar to the target value of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (azide
20 (azide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carboxylate).
carboxylate).
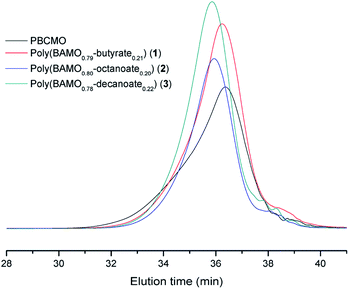
The dispersity values and the average molecular weights of poly(BAMO-carboxylate) compounds (1–3) were analyzed by GPC. The GPC chromatograms are shown in Fig. 3, and the detailed data are listed in Table 1. The substitution reaction gave high yields (90–92%), with average molecular weights ranging from 2080 g mol−1 to 2440 g mol−1, which represented an increase from the average molecular weight of PBCMO (1850 g mol−1) and polydispersity index (PDI = Mw/Mn) values that ranged from 1.22 to 1.30. The PDI values of all the poly(BAMO-carboxylate) compounds (1–3) are close to 1.00, indicating that the copolymers were successfully synthesized.
2.2 Thermal properties
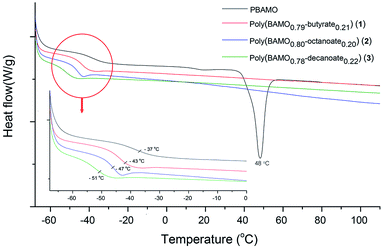
Thermal stability and low glass transition temperature are critical factors for increasing propellant insensitivity. DSC and TGA experiments were performed to determine the thermal properties. To confirm the effect of carboxylate substitution, the DSC and TGA experiments of pure PBAMO were also performed. The obtained values are summarized in Table 1. The three poly(BAMO-carboxylate) (1–3) have similar Tg values ranging from −43 °C to −51 °C (Table 1 and Fig. 4). In Fig. 4, a melting temperature (48 °C) was observed only in PBAMO.18The Tg values of the three poly(BAMO-carboxylate) were lower than that of PBAMO, and the longer the alkyl chain is, the lower glass transition temperature is. Partial introduction of long-chain carboxylates into PBAMO reduces the intramolecular and intermolecular forces in the skeleton, thereby lowering the Tg.31 In addition, it is expected that Tg is lowered because the symmetric structure of PBAMO is broken and the crystallinity is lowered due to random substitution of carboxylate. This property facilitates processability in the preparation of the propellant binder.6,32
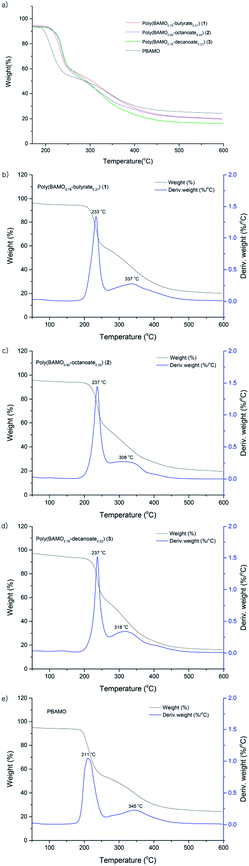
The thermal decomposition temperatures (Tdec) of compounds 1–3 were measured by TGA to determine their thermal stabilities. As shown in Fig. 5, there are two main exothermic peaks in compounds 1–3, ranging from 233 to 237 °C and from 308 to 337 °C, attributed to the energy release due to azido-decomposition forming N2 gas and the decomposition of polymer backbone. The compounds 1–3 have similar thermal decomposition temperatures that are about 20 °C higher than that of PBAMO (211 °C), i.e. 1–3 have better thermal stabilities than PBAMO.
2.3 Energetic properties
Besides thermal stability, sensitivity against stimuli and energetic properties is also important for the application of polymeric binders. The friction and impact sensitivities of synthesized copolymers were measured by NATO STANAG 4487 and NATO STANAG 4489, respectively.As shown in Table 2, the friction sensitivities of all the compounds was above the detection limit, and thus could not be compared. Compounds 1–3 have lower impact sensitivities than PBAMO, because the azide contents of compounds 1–3 were lower than that of PBAMO. The impact sensitivity of 1 was 38 J, which is higher than that of PBAMO (14 J). Notably, the impact sensitivities of 2 and 3, having longer alkyl chains, are above the detection limit of 50 J. Therefore, the properties of PBAMO, which is difficult to handle because of its sensitivity, must be improved.
| Poly(BAMO0.79-butyrate0.21) (1) | Poly(BAMO0.80-octanoate0.20) (2) | Poly(BAMO0.78-decanoate0.22) (3) | PBAMO | |
|---|---|---|---|---|
| Friction sensitivity (N) | >360 | >360 | >360 | >360 |
| Impact sensitivity (J) | 38 | >50 | >50 | 14 |
To check the energetic properties and to improve sensitivity, the energies of combustion (ΔUcomb) of PBAMO, poly(BAMO0.80-octanoate0.20) (2), and poly(BAMO0.78-decanoate0.22) (3) were measured by a Parr Bomb Calorimeter 6200. Then, the combustion enthalpies (ΔHcomb) were calculated at 25 °C based on the combustion energies (ΔHcomb = ΔUcomb + ΔnRT; Δn = Δni(product, g) − Δni(reactant, g)). The enthalpies of formation (ΔfHo) can be obtained by using the following equation: ΔfHo(copolymer) = aΔfHo(CO2) + 0.5bΔfHo(H2O) − ΔcHo(copolymer) (for the composition CaHbNcOd). It is based on the Hess thermochemical cycle at 25 °C with the combustion reactions of the repeating unit, as shown in Scheme 1. Here, the heats of formation for H2O (l) and CO2 (g) are −286 and −394 kJ mol−1, respectively.31
As shown in Table 3, the combustion enthalpies are always negative because combustion is an exothermic reaction. The combustion enthalpies of compounds 2 and 3 have more negative values in than that of PBAMO, because they have larger repeating units (greater molar mass) and |ΔUcomb| than the latter. It suggests that compounds 2 and 3 release higher energies during the combustion process than PBAMO. Additionally, the enthalpies of formation of 2 and 3 are negative, while that of PBAMO is positive (0.06 kJ g−1), because they have higher carbons and hydrogen contents in the repeating unit. In this case, contributions of the CO2 and H2O terms in the equation for the enthalpies of formation increase beyond the ΔcHo term.33 Therefore, compounds 2 and 3 have negative formation enthalpies, which indicate the product is more stable than the constituent elements. In contrast, the positive formation enthalpy of PBAMO suggests the opposite.
| PBAMO | Poly(BAMO0.80-octanoate0.20) (2) | Poly(BAMO0.78-decanoate0.22) (3) | |
|---|---|---|---|
| Formula (repeating unit) | C5H8N6O | C8.2H14.2N4.8O1.8 | C9H15.6N4.8O1.8 |
| FW (repeating unit) [g mol−1] | 168.16 | 208.84 | 219.86 |
| −ΔUcomb [J g−1] | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 590 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 023 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716 716 |
| −ΔHcomb [kJ mol−1] | 3125 | 5226 | 5665 |
| ΔfHom [kJ mol−1] | 10.78 | −35.05 | −121.33 |
| ΔfHo [kJ g−1] | 0.06 | −0.17 | −0.55 |
3. Experimental
3.1 Materials and instruments
PBCMO was supplied by J. CHEM. Inc. Butanoic acid, decanoic acid, and octanoic acid were supplied by PMC KOREA CO., LTD. The other chemicals were purchased from commercial sources (Sigma Aldrich and TCI), and were used without further purification, unless specifically mentioned. For the quantitative analysis of the product, the inverse-gated decoupling 13C NMR spectra were obtained in DMSO with a Bruker NMR and zpig30 pulse program. The FT-IR spectra were collected on an Alpha FT-IR spectrometer from Bruker using a diamond ATR accessory in the wavenumber range 4000 to 400 cm−1. The friction and impact sensitivities were determined using NATO STANAG 4487 and NATO STANAG 4489, respectively. DSC was performed on a TA instruments Q 1000 using aluminum pans at the heating rate of 10 °C min−1 under nitrogen flow, in the temperature range from −70 °C to 110 °C. TGA was performed on a PERKIN ELMER TGA Q500 V6.2 Build 187 under nitrogen atmosphere at a heating rate of 5 °C min−1, from 25 °C to 600 °C. The molecular weights of the materials were determined by WATERS 515, using THF as the flow solvent at the flow rate of 1.0 mL min−1. The heat of combustion was determined by the Parr Bomb Calorimeter 6200.3.2 General procedure for the synthesis of potassium carboxylate
For the synthesis of potassium carboxylate, methanol (50 mL), carboxylic acid (110 mmol), and potassium hydroxide (100 mmol) were added to a 100 mL round-bottom flask. The reaction mixture was stirred at room temperature for 2 h. After evaporating methanol, the potassium carboxylate was filtered off, washed with hexane, and dried in the oven at 40 °C for 24 h.3.3 General procedure for the synthesis of PBAMO copolymers based on carboxylate
The PBAMO copolymers were synthesized by the reaction of PBCMO, sodium azide, and potassium carboxylate. PBCMO (3.10 g, 20 mmol) was dissolved in DMSO (20 mL) in a 100 mL round-bottom flask at 60 °C. Potassium carboxylate (8 mmol) was added and stirred at 120 °C for 24 h. Then, sodium azide (2.29 g, 35.2 mmol) was added to the reaction mixture and stirred at 120 °C for 48 h. After the completion of the reaction, the mixture was cooled and 50 mL of ethyl acetate was added. The organic layer was washed with distilled water (50 mL × 4) to remove DMSO, dried over MgSO4, filtered through a pad of Celite, and concentrated under reduced pressure.4. Conclusions
Poly(BAMO-carboxylate) compounds were synthesized by the substitution of poly[3,3-bis(3-chloromethyl)oxetane] (PBCMO) with potassium carboxylate and sodium azide in DMSO, and were characterized by FT-IR and 13C NMR spectroscopy. The molecular composition of poly(BAMO-carboxylate) compounds was confirmed by inverse-gated decoupling 13C NMR spectroscopy, and the average molecular weight was measured by GPC. The poly(BAMO-carboxylate) compounds were found to have molecular compositions similar to the theoretically calculated ones. The thermal properties were measured by DSC and TGA. The energy properties were determined in terms of the sensitivity and heats of combustion and formation. The poly(BAMO-carboxylate) compounds were found to have lower glass transition temperatures and higher thermal decomposition temperatures than PBAMO. Poly(BAMO0.80-octanoate0.20) and poly(BAMO0.78-decanoate0.22) have negative formation enthalpies and higher heats of combustion than PBAMO. Moreover, the impact sensitivities of poly(BAMO-carboxylate) compounds were lower than those of PBAMO. Consequently, poly(BAMO-carboxylate) compounds exhibited better thermal and energetic properties than PBAMO as a polymeric binder for solid propellants. These properties facilitate processability in the preparation of the propellant binder. Therefore, poly(BAMO-carboxylate) is a good candidate as a prepolymer for designing energetic polymeric binders.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Defense Acquisition Program Administration (DAPA 912575201) and the Korean Ministry of Education through the BK21-Plus project.Notes and references
- M. S. Eroğlu and O. Güven, Polymer, 1998, 39, 1173–1176 CrossRef.
- B. Gaur, B. Lochab, V. Choudhary and I. K. Varma, J. Macromol. Sci., Polym. Rev., 2003, 43, 505–545 CrossRef.
- E. Landsem, T. L. Jensen, F. K. Hansen, E. Unneberg and T. E. Kristensen, Propellants, Explos., Pyrotech., 2012, 37, 581–591 CrossRef.
- Y. M. Mohan, Y. Mani and K. M. Raju, Des. Monomers Polym., 2012, 9, 201–236 CrossRef.
- J. Deng, G. Li, M. Xia, Y. Lan and Y. Luo, J. Appl. Polym. Sci., 2016, 133, 43840 CrossRef.
- R. G. Sracer and D. M. Husband, Propellants, Explos., Pyrotech., 1991, 16, 167–176 CrossRef.
- Y. Wu, Y. Luo and Z. Ge, Propellants, Explos., Pyrotech., 2015, 40, 67–73 CrossRef CAS.
- M. Cappello, S. Filippi, L. Mori and G. Polacco, Propellants, Explos., Pyrotech., 2017, 42, 974–981 CrossRef CAS.
- Y. Zhou, X.-P. Long and Q.-X. Zeng, J. Appl. Polym. Sci., 2012, 125, 1530–1537 CrossRef CAS.
- I. K. Varma, Macromol. Symp., 2004, 210, 121–129 CrossRef.
- Y. M. Mohan, K. M. Raju and B. Sreedhar, Int. J. Polym. Mater., 2006, 55, 441–455 CrossRef.
- M. Cappello, S. Filippi, L. Mori and G. Polacco, Int. J. Polym. Mater., 2017, 42, 974–981 Search PubMed.
- A. M. Kawamoto, J. A. S. Holanda, U. Barbieri, G. Polacco, T. Keicher, H. Krause and M. Kaiser, Propellants, Explos., Pyrotech., 2008, 33, 365–372 CrossRef.
- Y. Zhang, J. Zhao, P. Yang, S. He and H. Huang, Polym. Eng. Sci., 2012, 52, 768–773 CrossRef.
- J.-F. Pei, F.-Q. Zhao, X.-D. Song, X.-N. Ren, H.-X. Gao, T. An, J. An and R.-Z. Hu, J. Anal. Appl. Pyrolysis, 2015, 112, 88–93 CrossRef.
- G. Li, H. Dong, M. Liu, M. Xia, C. Chai and Y. Luo, Polym. Int., 2017, 66, 1037–1043 CrossRef CAS.
- B. S. Min and S. W. Ko, Macromol. Res., 2007, 15, 225–233 CrossRef.
- S. Pisharath and H. G. Ang, Polym. Degrad. Stab., 2007, 92, 1365–1377 CrossRef.
- B. Li, Y. Zhao, G. Liu, X. Li and Y. Luo, J. Therm. Anal., 2016, 126, 717–724 CrossRef.
- M. Xu, Z. Ge, X. Lu, H. Mo, Y. Ji and H. Hu, RSC Adv., 2017, 7, 47271–47278 RSC.
- Y. M. Mohan and K. M. Raju, Int. J. Polym. Mater., 2006, 55, 203–217 CrossRef.
- Y. M. Mohan, M. P. Raju and K. M. Raju, Int. J. Polym. Mater., 2005, 54, 651–666 CrossRef.
- U. Barbieri, G. Polacco, T. Keicher and R. Massimi, Propellants, Explos., Pyrotech., 2009, 34, 427–435 CrossRef CAS.
- J.-F. Pei, F.-Q. Zhao, H.-L. Lu, X.-D. Song, R. Zhou, Z.-F. Yuan, J. Zhang and J.-B. Chen, J. Therm. Anal., 2016, 124, 1301–1307 CrossRef CAS.
- H. Kim, Y. Jang, S. Noh, J. Jeong, D. Kim, B. Kang, T. Kang, H. Choi and H. Rhee, RSC Adv., 2018, 8, 20032–20038 RSC.
- Y. Oyumi and T. B. Brill, Combust. Flame, 1986, 65, 127–135 CrossRef.
- C. Zhang, J. Li, Y.-J. Luo and B. Zhai, J. Energ. Mater., 2015, 33, 305–314 CrossRef.
- K. E. Hardenstine, G. V. S. Henderson, L. H. Sperling, C. J. Murphy and G. E. Manser, J. Polym. Sci., Part B: Polym. Phys., 1985, 23, 1597–1609 Search PubMed.
- M. Cao, T. Li, J. Liang and G. Du, Polymers, 2017, 9, 109 CrossRef PubMed.
- M. Cao, T. Li, J. Liang, Z. Wu, X. Zhou and G. Du, Polymers, 2016, 8, 391 CrossRef PubMed.
- S. Hafner, T. Keicher and T. M. Klapötke, Propellants, Explos., Pyrotech., 2017, 43, 126–135 CrossRef.
- J. Blackwell, M. R. Nagarajan and T. B. Hoitink, Polymer, 1982, 23, 950–956 CrossRef CAS.
- E. Diaz, P. Brousseau, G. Ampleman and R. E. Prud'homme, Propellants, Explos., Pyrotech., 2003, 28, 101–106 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |