Open Access Article
Open Access ArticleA promising cation of 4-aminofurazan-3-carboxylic acid amidrazone in desensitizing energetic materials†
Jichuan Zhang‡
ac,
Zhenyuan Wang‡b,
Yunhao Hsiehb,
Binshen Wangb,
Haifeng Huang a,
Jun Yang
a,
Jun Yang *a and
Jiaheng Zhang
*a and
Jiaheng Zhang *bc
*bc
aCAS Key Laboratory of Energy Regulation Materials, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032, China. E-mail: yangj@sioc.ac.cn
bSchool of Material Sciences and Engineering, Harbin Institute of Technology, Shenzhen, 518055, China. E-mail: zhangjiaheng@hit.edu.cn
cBiomaterials Research Center, Zhuhai Institute of Advanced Technology, Chinese Academy of Sciences, Zhuhai, 519003, China
First published on 14th January 2020
Abstract
For the development of energetic materials, insensitive compounds have attracted considerable attention due to their improved safety and lower cost than those of sensitive energetic compounds during production, transportation, and application. In this study, insensitive 4-aminofurazan-3-carboxylic acid amidrazone was used as a cation to obtain four derivatives which were determined by X-ray single crystal diffraction. It is interesting to note that all four derivatives are insensitive to impact and friction, while the velocities of detonation for derivatives are superior to that of insensitive TATB (1,3,5-triamino-2,4,6-trinitrobenzene). Multi-factors analysis shows that the cation of 4-aminofurazan-3-carboxylic acid amidrazone is a promising furazan-based cation in desensitizing energetic compounds.
Introduction
Since the discovery of dynamite by Alfred Bernhard Nobel, energetic compounds with a considerable amount of stored chemical energy have played a crucial role in the civil and military defence industries.1 During the development and investigation of new-generation energetic compounds, insensitive energetic materials have attracted extensive attention as it can decrease the cost and risk greatly compared to those of less sensitive or sensitive compounds during the production, transportation, and application.2 Several strategies were employed to design insensitive compounds, such as introducing insensitive groups (NH2, CH3) to energetic backbone, assembly of energetic metal–organic frameworks, layer–layer packing, forming strong hydrogen bonds, as well as the combination of the above multi-factors.3Hydrogen bonds (H-bonds) are divided into two categories, intramolecular and intermolecular H-bonds,4 which play a significant role in desensitization of energetic compounds. In general, intramolecular H-bonds are the stronger one, this is due to (1) intramolecular H-bonds connect donator and acceptor in a molecule, which can make the molecule considerably more firm than those contain intramolecular H-bonds; and (2) intramolecular H-bonds could enhance the conjugation effect of the molecule, and result in a planar molecule, which are usually less sensitive than nonplanar energetic compounds, such as the classic insensitive compounds, TATB, LLM-116, and FOX-7, which are obviously less sensitive than their derivatives.3b,5 However, for energetic materials, most of the reported strong intramolecular H-bonds are N–H⋯O, the intramolecular H-bonds of N–H⋯N are reported rarely.4a In addition, intermolecular H-bonds help form infinite networks as it may also cause denser packing which reduces the amount of free space in the crystal lattice, and thereby decreasing sensitivity.6
Nitrogen-rich heterocyclic rings have drawn extensive interest in the past two decades due to their high heat of formation and nitrogen content.7 Comparing with these general known heterocyclic rings, 1,2,5-oxadiazole ring (furazan) exhibits promising performance due to its higher density, and better oxygen balance than pyrazole and 1,2,4-triazole. In addition, furazan also displays higher heat of formation than 1,2,4-oxadiazole ring.8 Some derivatives with furazan ring show huge potential as new generation energetic materials.9 The cations of furazan based energetic salts are inorganic cations, such as ammonium, hydronium, and hydroxylammonium. Although some azole rings have been employed as energetic cations while the furazan ring as energetic cation has been studied rarely.
4-Aminofurazan-3-carboxylic acid amidrazone which is insensitive to impact and friction was synthesized and characterized in this work. Single crystal structure shows that it contains not only intermolecular H-bonds, but also strong N⋯H–N intramolecular H-bonds. If these H-bonds were still in its derivatives, the sensitivities of its derivatives would possibly be decreased greatly. Now, four derivatives (1a: nitrate; 1b: 1H,1H-5,5-bitetrazole-1,1-diolate; 1c: 3-nitramino-4-tetrazolefurazanate; 1d: 3,4-dinitramino-furazanate) of 4-aminofurazan-3-carboxylic acid amidrazone were synthesized and characterized (Scheme 1). Their crystal structures, energetic properties, and sensitivities were investigated comprehensively.
Results and discussion
Synthesis and single crystal X-ray structures
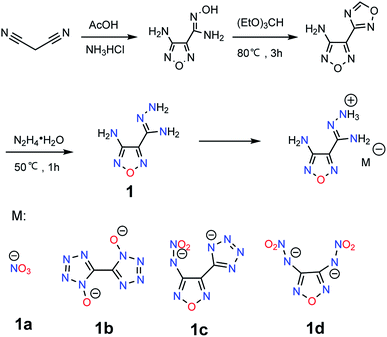
Based on the previous study10 and the synthetic route shown in Fig. 1, 4-(1,2,4-oxadiazol-3-yl)furazan-3-amine, a luminous yellow solid, was synthesized in high yield (85%), followed by treatment with 80% of hydrazine hydrate in methanol at 50 °C for 1 h while 4-aminofurazan-3-carboxylic acid amidrazone (1) exhibits as a brown solid. Compound 1 was recrystallized from water, and then it reacted with dilute HNO3 in methanol, after the solvent was removed, affording 1a·H2O. 1 reacted with HCl, affording an amidrazone of 4-aminofurazan-3-carboxylic acid chloride salt and then chloride salt of 1 react with the corresponding silver salts of 1b–1d in water for 30 min, 1b–1d were obtained after filtration, evaporation. Finally, these four salts were obtained after recrystallization from mixture of water and methanol, and drying, respectively.Compound 1 was crystalized from water in the monoclinic space group P121/c1 with a density of 1.627 g cm−3 at 173 K (Table S1†), as well as eight molecules in a unit cell (Z = 8, Fig. 2a). Five intramolecular H-bonds were observed in 1: N9–H9B⋯N11, N4–H4A⋯N1, N3–H3B⋯N5, N10–H10A⋯N12 and N10–H10B⋯N7. The corresponding hydrogen⋯acceptor (H⋯A) distances were 2.215 Å, 2.332 Å, 2.410 Å, 2.425 Å and 2.410 Å (Table 1), respectively. Comparing to the reported N–H⋯N intramolecular H-bonds (H⋯A > 2.52 Å),4a the intramolecular H-bonds in 1 are shorter and stronger while the amount of intramolecular H-bonds is greater. The intramolecular H-bonds resulted in coplanar structure of all the non-hydrogen atoms of 1 (Fig. 4a and Tables S1 and S2†). Apart from intramolecular H-bonds, five intermolecular H-bonds were also observed, with lengths of 2.209, 2.219, 2.335, 2.425, and 2.465 Å, respectively (Table S6†). Finally, the crystal structure was connected by these intermolecular H-bonds, forming an infinite three-dimensional (3D) network (Fig. S6 and 7†).
 | ||
| Fig. 2 Single crystal structures and hydrogen bonds of 1 (a) and 1a·H2O (b) (hydrogen bonds: green dash line). | ||
| Comp | D–H⋯A | D–H (Å) | H⋯A (Å) | D–H⋯A (Å) | <DHA (°) |
|---|---|---|---|---|---|
| 1 | N3–H3B⋯N5 | 0.883 | 2.410 | 2.930 | 118.06 |
| N9–H9⋯N11 | 0.881 | 2.215 | 2.814 | 124.91 | |
| N4–H4A⋯N1 | 0.884 | 2.332 | 2.798 | 112.97 | |
| N10–H10B⋯N7 | 0.886 | 2.425 | 2.799 | 105.78 | |
| N10–H10A⋯N12 | 0.886 | 2.419 | 2.699 | 98.72 | |
| 1a·H2O | N3–H3B⋯N4 | 0.805 | 2.295 | 2.847 | 126.37 |
| N6–H6A⋯N1 | 0.861 | 2.382 | 2.771 | 107.89 | |
| 1b | N7–H7B⋯N8 | 0.859 | 2.269 | 2.826 | 122.54 |
| 1c | N3–H3B⋯N5 | 0.882 | 2.168 | 2.855 | 134.36 |
| N9–H9B⋯N11 | 0.880 | 2.250 | 2.828 | 123.02 | |
| N4–H4B⋯N2 | 0.880 | 2.455 | 2.770 | 102.31 | |
| N6–H6A⋯N4 | 0.910 | 2.491 | 2.727 | 95.12 | |
| N10–H10B⋯N8 | 0.880 | 2.471 | 2.794 | 102.30 |
Compound 1a·H2O was crystalized as a colourless block crystal in the orthorhombic Pbca space group, with eight NO3 anions, eight amidrazone of 4-aminofurazan-3-carboxylic acid cations, and eight water molecules in each unit cell (Z = 8, Fig. 2b). The crystal density of 1a·H2O was 1.64 g cm−3 at room temperature. It is interesting that the intramolecular H-bonds still exist in 1a·H2O, and two intramolecular H-bonds (N3–H3B⋯N4) and (N6–H6A⋯N1) are observed. The hydrogen⋯acceptor (H⋯A) distance are 2.295 Å and 2.382 Å, respectively. The intramolecular H-bonds make the atoms of cation and oxygen from water molecules close to coplanar (Fig. 4b and Table S3†). In addition, seven intermolecular H-bonds were observed in a unit cell, and the lengths of their H⋯A were 1.881, 2.010, 2.077, 2.082, 2.198, 2.203 and 2.209 Å, respectively. These intermolecular H-bonds connect NO3 anions, water molecules as well as amidrazone of 4-aminofurazan-3-carboxylic acid cations forming an infinite 3D network structure (Fig. S8 and 9†).
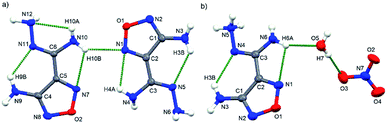
1b was crystalized as a colourless block in the monoclinic P21/c space group accompanied by four anions and eight cations in a unit cell (Fig. 3a). Its density was 1.70 g cm−3 at room temperature. The structure of 1b is similar to 1a as 1b contains intramolecular H-bond (N7–H7B⋯N8) in the cation while the hydrogen⋯acceptor (H7B⋯N8) distance was 2.275 Å. The intramolecular H-bonds cause all the non-hydrogen atoms of the cation to coplanar (Fig. 4c and Table S4†). Moreover, a large amount of intermolecular H-bonds was observed throughout the intermolecular H-bonds connected 3D crystal structure (Fig. S10 and 11†), with lengths of 1.892, 2.048, 2.119, 2.303, 2.271, and 2.489 Å, respectively.
 | ||
| Fig. 3 Single crystal structures and hydrogen bonds of 1b (a) and 1c (b) (hydrogen bonds: green dash line). | ||
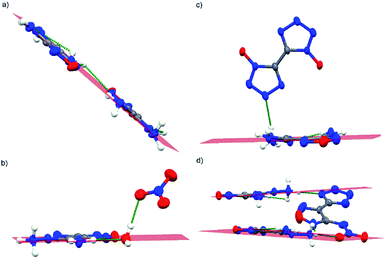 | ||
| Fig. 4 Coplanar structures of 1 and cations in various compounds ((a): compound 1; (b): compound 1a; (c): compound 1b; (d): compound 1c). | ||
1c was crystalized in the monoclinic P1n1 space group, with two anions and four cations in a unit cell (Z = 2, Fig. 3b). Similar to 1, five intramolecular H-bonds (N3–H3B⋯N5, N9–H9B⋯N11, N4–H4B⋯N2, N6–H6A⋯N4 and N10–H10B⋯N8 respectively) were observed in two neighbouring cations, and their corresponding hydrogen⋯acceptor (H3B⋯N5, H9B⋯N11) lengths were 2.168 Å, 2.250 Å, 2.455 Å, 2.471 Å and 2.491 Å, respectively, which are more stronger than those in 1. The intramolecular H-bonds make cation almost coplanar (Fig. 4d and Table S5†). Further, as many as 13 intermolecular H-bonds throughout the crystal were observed, and the hydrogen⋯acceptor (H⋯A) lengths of these intermolecular H-bonds were 1.928, 1.983, 2.030, 2.060, 2.120, 2.183, 2.193, 2.295, 2.311, 2.355, 2.457, 2.491 and 2.499 Å, respectively. The crystal structure was connected by these 13 intermolecular H-bonds, forming an infinite 3D network (Fig. S12 and 13†).
To conclude, these N–H⋯N intramolecular H-bonds caused all of the non-hydrogen atoms in the neutral compound and cations coplanar (Fig. 4). The hydrogen⋯acceptor (H⋯A) distance of these intramolecular H-bonds ranged from 2.168 Å to 2.491 Å; they are not only shorter than those reported N–H⋯N intramolecular H-bonds (>2.52 Å), but also shorter than most of those N–H⋯N intermolecular H-bonds (H⋯A: 2.187–2.345 Å).4a,11 Hence, these strong intramolecular H-bonds permit the close stacking of these compounds. Additionally, the existing large amounts of intermolecular H-bonds of the corresponding compounds allow compounds to pack closely and further led to infinite 3D networks, particularly 1c, which displayed firm structure as 13 intermolecular H-bonds were observed throughout the crystal.
Thermal stability and sensitivity
Thermal stability is a crucial factor for energetic compounds: the higher the decomposition temperature, the safer the energetic compounds. Thermogravimetric analysis-differential scanning calorimetry (DSC, 5 °C min−1) was employed to characterize the thermal stability of all five compounds prepared in this study. The decomposition temperatures (on set) of 1 was 183.4 °C, while the disintegration temperature of 1a–1d were 133.0, 184.9, 183.3, and 198.5 °C (Fig. S1–S5†), respectively. The decomposition temperatures of four compounds, 1, 1b, 1c and 1d, were greater than 180 °C.All five samples were examined by the Fall-hammer and Friction apparatus and all of them exhibited insensitivity (IS > 40 J, FS > 360 N) to stimulation via impact and friction.19 Hence, it is beneficial to investigate the insensitivity of these compounds. In this study, all crystals prepared contain N–H⋯N intramolecular H-bonds. As comparing examples with 1a, 3,5-diamino-1,2,4-triazole, 4,4′,5,5′-tetraamino-3,3′-bis-1,2,4-triazole and 3-methyl-1-amino-1,2,3-triazole are insensitive. The impact and friction sensitivities of 3,5-diamino-1,2,4-triazole nitrate salts are 40 J and 288 N, respectively, and the impact sensitivities of 3-methyl-1-amino-1,2,3-triazole nitrate and 4,4′,5,5′-tetraamino-3,3′-bis-1,2,4-triazole nitrate are 28.9 J and 15 J, respectively, which are obviously more sensitive than those of 1a,12 which would possibly result from the strong intramolecular H-bonds, and large amount of intermolecular H-bonds in the structures. Similarly, compounds 1b and 1c were less sensitive than the energetic compounds based on 5,5′-dihydrobistetrazole and 3-nitramino-4-tetrazolefurazan.9d,13 Thus far, all compounds based on 3,4-di(nitramino)furazan were sensitive; hence, 1d can offer a new route to desensitize compounds of 3,4-di(nitramino)furazan.9a
In general, the “soft” O–H and N–H interactions are conducive to stabilizing the crystal structure by absorbing the mechanic stimuli.14 In this study, Hirshfeld surfaces15 were employed to analyse the weak interactions of the obtained compounds. As shown in Table 2, the percentage of O–H weak interactions in 1 and 1a·H2O, 1b–1c are 13.2%, 53.1%, 23.4% and 15.7%, respectively, while the percentage of N–H weak interactions in 1, 1a·H2O and 1b–1c are 35.0%, 15.4%, 38.4%, and 38.2%, respectively. The proportion of N–H weak interactions is higher than O–H weak interactions except 1a·H2O, which may due to more O atoms provided by NO3 anion and H2O in 1a·H2O. The strength of N–H weak interactions in the case of 1 and 1c are also higher than the strength of O–H weak interactions as shown in Fig. 5 (Fig. S14†). The investigation of weak interactions displays that the large portions of N–H weak interactions are possibly also helpful in decreasing sensitivities.
| Crystal | 1 | 1a·H2O | 1b | 1c |
|---|---|---|---|---|
| O–H (%) | 13.2 | 53.1 | 23.4 | 15.7 |
| N–H (%) | 35.0 | 15.4 | 38.4 | 38.2 |
Detonation properties
To examine the energetic properties of 1 and its derivatives, the heat formation for 1 and its corresponding anion were determined by the isodesmic reaction approach (see ESI†). The computation was performed using the Gaussian 03 suite of programs.17 The heat formation for 1 and 1a–1d were 329.1, 208.2, 1507.2, 1432.5, and 1206.1 kJ mol−1, respectively (Table 3). The detonation properties of all new compounds were calculated by EXPLO5 6.01 code with the calculated enthalpies of formation and the measured densities.18 The detonation velocities and pressures of 1 and 1a–1d were 7999, 8455, 8680, 8249, and 8541 m s−1 and 22.20, 27.73, 28.77, 25.57, and 28.42 GPa (Table 2), respectively. The detonation properties of all the compounds were greater than that of TNT; especially, the detonation velocity of 1b, which exhibited the best detonation performance, was 8680 m s−1. This was greater than LLM-116 and comparable to that of RDX. The detonation velocity of 1d was greater than that of TATB and comparable to that of LLM-116. Samples 1a, 1b, and 1d demonstrated immense applications as alternatives to traditional classic compounds as they exhibited moderate detonation velocities at an insensitive level.| Comp. | Tma (°C) | Tda b (°C) | db c (g cm−3) | ΔfHc d (kJ mol−1) | Vdd e (m s−1) | Pe f (Gpa) | ISf g (J) | FSg h (N) |
|---|---|---|---|---|---|---|---|---|
| a Melting point.b Temperature of decomposition (onset).c Density measured by using a gas pycnometer at 25 °C.d Calculated molar enthalpy of formation in the solid state.e Calculated detonation velocity.f Calculated detonation pressure.g Impact sensitivity.h Friction sensitivity.i Ref. 3a and 16. | ||||||||
| 1 | 167.3 | 198.4 | 1.60 | 329.05 | 7999 | 22.20 | >40 | >360 |
| 1a | 128.5 | 133 | 1.68 | 208.2 | 8455 | 27.73 | >40 | >360 |
| 1b | 81.6 | 184.9 | 1.70 | 1507.2 | 8680 | 28.77 | >40 | >360 |
| 1c | 85.7 | 183.3 | 1.64 | 1432.5 | 8249 | 25.57 | >40 | >360 |
| 1d | 106.4 | 198.5 | 1.68 | 1206.06 | 8541 | 28.42 | >40 | >360 |
| TATBi | — | 360 | 1.93 | −154.2/−0.60 | 8114 | 31.2 | >40 | >360 |
| LLM-116i | — | 178 | 1.90 | 96.3/0.56 | 8573 | 34.2 | >40 | >360 |
| RDXi | — | 205 | 1.81 | 80/0.36 | 8795 | 34.9 | 7.4 | 120 |
| TNTi | 81 | 295 | 1.65 | −67.0/−0.30 | 6881 | 19.5 | 15 | 353 |
Conclusions
Insensitive 4-aminofurazan-3-carboxylic acid amidrazone (1) was synthesized, and then its four derivatives (1a–1d) were prepared and characterized. It is interesting that all four derivatives are insensitive to impact and friction, which were resulted from the combination of strong N–H⋯N intramolecular H-bonds, a large amount of intermolecular H-bonds, and high percentage of weak N–H interactions in these derivatives. In addition, the detonation velocity of compound 1b is higher than that of LLM-116 and comparable to that of RDX. The detonation velocity of 1d is greater than that of TATB and comparable to that of LLM-116 demonstrating their promising for applications. In a word, combining multi-stabilizing factors, 4-aminofurazan-3-carboxylic acid amidrazone cation is promising in desensitizing energetic materials.Experimental
Safety precautions
Although we experienced no difficulties in synthesis and characterization of these materials, proper protective procedures should be followed. All the experiments were carried out in a hood behind a safety shield and face shield, and leather gloves were worn at all times. Caution was exercised at all times during the synthesis, characterization, and handling of any of these materials.Materials and methods
1H spectra were recorded on a 300 MHz nuclear magnetic resonance spectrometer operating at 300 MHz. 13C NMR spectra were recorded on a 400 MHz nuclear magnetic resonance spectrometer. The solvent were [D6]dimethylsulfoxide ([D6]DMSO) and [D4]methanol ([D4]CH3DO) unless otherwise specified. 15N NMR spectra was recorded on a 400 MHz nuclear magnetic resonance spectrometer operating at 40.5 MHz, the 13C and 15N spectrums were showed from Fig. S15–S20.† Except for single crystal structure determination, before all characterizations (such as thermal stability, sensitivities, element analysis and density, etc.), the samples were dried in 60 °C for 6 h, in order to remove the water or moisture of the samples. The melting and decomposition points were recorded on a NETZSCH STA 449F3 equipment at a scan rate of 5 °C min−1, respectively. Infrared spectra were recorded by using KBr pellets. Densities were measured at room temperature using a Micromeritics Accupyc II 1340 gas pycnometer. Elemental analyses were obtained on an Elementar Vario MICRO CUBE (Germany) elemental analyser. Impact and friction-sensitivity measurements were tested by employing a standard BAM Fallhammer and a BAM friction tester. The acknowledgements come at the end of an article after the conclusions and before the notes and references.X-ray crystallography
The single-crystal X-ray diffraction data of 1 and 1a–1c were collected at 293 K using graphite-monochromated MoKα radiation (λ = 0.71073 Å) using omega scans from a Bruker CCD area detector diffractometer. Data collection and reduction were performed, and the unit cell was initially refined using Bruker SMART software.20 The reflection data were also corrected for LP factors. The structure was solved by direct methods and refined by a least-squares method on F2 using the Bruker SHELXTL program.21 In these structures, the value of the Flack parameter did not allow the direction of the polar axis to be determined and Friedel reflections were then merged for the final refinement. Details of the data collection and refinement are given in Table S1.†Computational method
The gas phase heats of formation for all new neutral compounds and anions were obtained using isodesmic reactions. The geometric optimization and frequency analysis of the structures were calculated using B3LYP function with 6-31+G** basis set. All of the optimized structures were checked to be true local energy minima on the potential energy surface without imaginary frequencies. Single-point energies based on the optimized structures were calculated at the MP2/6-311++G* set. Atomization energies for the frame molecules or ions were obtained by employing the G2 ab initio method. The conversion of gas phase enthalpies to solid phase values for the neutral compounds was done by subtracting the empirical heat of sublimation obtained based on Trouton's rule. Detonation properties of new compounds were calculated by using EXPLO5 program. More calculation details can be found in the ESI.†Syntheses
According to the literature10 and synthesis route depicted in Fig. 1, 4-(1,2,4-oxadiazol-3-yl) furazan-3-amine (1.53 g, 10 mmol), a luminous yellow solid, was synthesized with high yield and then treated with 80% hydrazine hydrate in methanol at 50 °C for 30 min. Hence, amidrazone of 4-aminofurazan-3-carboxylic acid (1) was obtained as brown solid with high yield, compound 1 was recrystallized from water, the brown needle-like crystal was obtained, which were used for the synthesis of salts. Yellow solid (1.35 g, 95.3%); 1H NMR (DMSO-d6): δ 6.39 (NH2, 2H), 5.86 (NH2, 2H), 569 (NH2, 2H) ppm; 13C NMR (DMSO-d6): δ 155.01, 141.10, 137.13 ppm; 15N NMR ([D4]CH3DO): δ 17.04, −21.67, −122.26, −282.99, −316.14, 335.19 ppm; IR (KBr): ν 3469, 3415, 3353, 3335, 2918, 2848, 1662, 1611, 1555, 1466, 1440, 1405, 1323, 1194, 1144, 1050, 992, 870, 841, 787, 754, 721, 574 cm−1; elemental analysis, calcd (%) for C3H6N6O (142.06): C: 25.35; H: 4.26; N: 59.13; found, C: 25.18; H: 3.98; N: 58.73.General procedure for the preparation of salts 1a
Compound 1 (0.142 g, 1 mmol) was added to methanol (20 ml) with stirring. HNO3 (5%, 1.23 ml, 1 mmol) was added into the above methanol. The reaction mixture was stirred at room temperature for 30 min followed by evaporation in vacuo. The precipitate was collected and recrystalized in the methanol and water. Colourless crystal contains one molecular water on each crystal unit cell (0.18 g, 80.7%), after drying in 60 °C for 6 h, the water in molecular were removed. The 1H NMR (DMSO-d6): δ 9.29 (NH3, 3H), 8.08 (NH2, 2H), 6.42 (NH2, 2H) ppm; 13C NMR (DMSO-d6): δ 155.49, 152.77, 140.23 ppm; IR (KBr): ν 3064, 1692, 1545, 1481, 1449, 1351, 1222, 1215, 1150, 1065, 1036, 955, 802, 760, 739, 548 cm−1; elemental analysis, calcd (%) for C3H7N7O4 (204.13): C: 17.65; H: 2.96; N: 48.03; found, C: 17.29; H: 3.06; N: 47.83.General procedure for the preparation of salts 1b–1d
To a stirring solution of 1·HCl (0.353 mg, 2.0 mmol), the silver compounds (1.0 mmol) of 1b, 1c and 1d were added to the above solutions, respectively. Then, the reaction mixture was stirred at room temperature for 30 min followed by filtration, evaporation. The precipitates were recrystalized from the mixture of methanol and water, respectively. Then dried at 60 °C for 6 h for the next characterisations.Amidrazone of 4-aminofurazan-3-carboxylic acid 5,5′-bistetrazole-1,1′-diolate (1b)
Yellow solid (0.42 g, 93.1%); 1H NMR (DMSO-d6): δ 6.92 (NH3, 3H), 6.39 (NH2, 2H), 5.91 (NH2, 2H) ppm; 13C NMR (DMSO-d6): δ 155.26, 144.56, 140.71, 135.59 ppm; IR (KBr): ν 3064, 1692, 1545, 1481, 1449, 1351, 1222, 1215, 1150, 1065, 1036, 955, 802, 760, 739, 548 cm−1; elemental analysis, calcd (%) for C8H14N20O4 (454.15): C: 21.15; H: 3.11; N: 61.66; found, C: 20.83; H: 2.76; N: 61.55.Amidrazone of 4-aminofurazan-3-carboxylic acid 3-nitramino-4-tetrazolefurazanate (1c)
Yellow solid (0.44 g, 91.0%); 1H NMR (DMSO-d6): δ 7.02 (NH2–NH3, 5H), 6.39 (NH2, 2H) ppm; 13C NMR (DMSO-d6): δ 158.06, 155.34, 147.64, 145.50, 141.87, 140.72 ppm; IR (KBr): ν 3343, 3287, 3074, 2992, 2887, 1725, 1628, 1575, 1508, 1250, 1140, 1102, 1080, 966, 746 cm−1; elemental analysis, calcd (%) for C9H14N20O5 (482.15): C: 22.41; H: 2.93; N: 58.08; found, C: 22.07; H: 3.17; N: 57.89.Amidrazone of 4-aminofurazan-3-carboxylic acid 3,4-dinitraminofurazanate (1d)
Yellow solid (0.43 g, 91.5%); 1H NMR (DMSO-d6): δ 7.12 (NH3, 3H), 6.92 (NH2, 2H), 6.41 (NH2, 2H) ppm; 13C NMR (DMSO-d6): δ 155.69, 155.24, 147.65, 141.85, 140.01 ppm; IR (KBr): ν 3466, 3414, 3349, 3269, 3206, 1723, 1647, 1494, 1452, 1361, 1219, 1134, 1072, 962 cm−1; elemental analysis, calcd (%) for C8H14N18O7 (474.13): C: 20.26; H: 2.98; N: 53.13; found, C: 20.15; H: 2.63; N: 53.40.Author contributions
Yunhao Hsieh polished this paper. Binshen Wang and Haifeng Huang helped do some calculations for this job. Jun Yang and Jiaheng Zhang provide the idea of this paper together.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (NSFC, Grant. No. 21602241), Shenzhen Science and Technology Innovation Committee (JCYJ20151013162733704), Economic, Trade and Information Commission of Shenzhen Municipality through the Graphene Manufacture Innovation Center (201901161514), and the Thousand Talents Plan (Youth).References
- (a) J. P. Agrawal and R. D. Hodgson, Organic Chemistry of Explosives, John Wiley & Sons Ltd, Copyright, 2007 Search PubMed; (b) H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- (a) L. Hu, P. Yin, G. Zhao, C. He, G. H. Imler, D. A. Parrish, H. Gao and J. M. Shreeve, J. Am. Chem. Soc., 2018, 140, 15001–15007 CrossRef CAS PubMed; (b) W. Zhang, J. Zhang, M. Deng, X. Qi, F. Nie and Q. Zhang, Nat. Commun., 2017, 8, 181 CrossRef; (c) Y. Tang, D. Kumar and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 13684–13687 CrossRef CAS PubMed; (d) J. Zhang, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2018, 6, 22705–22712 RSC; (e) Q. Wang, S. Wang, X. Feng, L. Wu, G. Zhang, M. Zhou, B. Wang and L. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 37542–37547 CrossRef CAS PubMed; (f) Y. Zhang, H. Gao, Y.-H. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2011, 50, 9554–9562 CrossRef CAS PubMed.
- (a) J. Zhang, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2018, 6, 22705–22712 RSC; (b) J. Zhang, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 10532–10535 CrossRef CAS PubMed; (c) Y. Wang, Y. Liu, S. Song, Z. Yang, X. Qi, K. Wang, Y. Liu, Q. Zhang and Y. Tian, Nat. Commun., 2018, 9, 2444 CrossRef PubMed; (d) S. Li, Y. Wang, C. Qi, X. Zhao, J. Zhang, S. Zhang and S. Pang, Angew. Chem., Int. Ed., 2013, 52, 1–6 CrossRef.
- (a) C. He, P. Yin, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Chem. Commun., 2016, 52, 8123–8126 RSC; (b) Y. Ma, A. Zhang, C. Zhang, D. Jiang, Y. Zhu and C. Zhang, Cryst. Growth Des., 2014, 14, 4703–4713 CrossRef CAS.
- (a) P. A. Pagoria, Propellants, Explos., Pyrotech., 2016, 41, 452–469 CrossRef CAS; (b) Y. Tang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, Dalton Trans., 2019, 48, 7677–7684 RSC; (c) R. D. Schmidt, G. S. Lee, P. F. Pagoria and A. R. Mitchell, J. Heterocycl. Chem., 2001, 38, 1227–1230 CrossRef CAS; (d) N. V. Muravyev, A. A. Bragin, K. A. Monogarov, A. S. Nikiforova, A. A. Korlyukov, I. V. Fomenkov, N. I. Shishov and A. N. Pivkina, Propellants, Explos., Pyrotech., 2016, 41, 999–1005 CrossRef CAS; (e) H. Gao and J. M. Shreeve, Angew. Chem., Int. Ed., 2015, 54, 6335–6338 CrossRef CAS PubMed.
- T. Brinck, Green Energetic Materials, Wiley, 2014 Search PubMed.
- P. Yin, Q. Zhang and J. M. Shreeve, Acc. Chem. Res., 2016, 49, 4–16 CrossRef CAS PubMed.
- J. Zhang, P. Yin, G. Pan, Z. Wang, J. Zhang, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, New J. Chem., 2019, 43, 12684–12689 RSC.
- (a) Y. Tang, J. Zhang, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 15984–15987 CrossRef CAS PubMed; (b) C. He and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 55, 772–775 CrossRef CAS PubMed; (c) Y. Li, H. Huang, Y. Shi, J. Yang, R. Pan and X. Lin, Chem.–Eur. J., 2017, 23, 7353–7360 CrossRef CAS PubMed; (d) H. Huang, Y. Shi, Y. Li, Y. Liu and J. Yang, RSC Adv., 2016, 6, 64568–64574 RSC.
- (a) A. I. Stepanov, V. S. Sannikov, D. V. Dashko, A. G. Roslyakov, A. A. Astrat’ev and E. V. Stepanova, Chem. Heterocycl. Compd., 2015, 51, 350–360 CrossRef CAS; (b) A. I. Stepanov, V. S. Sannikov, D. V. Dashko, A. G. Roslyakov, A. A. Astrat’yev, E. V. Stepanova, Z. G. Aliev, T. K. Goncharov and S. M. Aldoshin, Chem. Heterocycl. Compd., 2017, 53, 779–785 CrossRef CAS.
- (a) C. Zhang, C. Sun, B. Hu, C. Yu and M. Lu, Science, 2017, 355, 374–376 CrossRef CAS PubMed; (b) Y. Zhang, Y. Huang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem., 2011, 21, 6891–6897 RSC.
- (a) Q. Lin, Y. Li, Y. Li, Z. Wang, W. Liu, C. Qi and S. Pang, J. Mater. Chem., 2012, 22, 666–674 RSC; (b) T. M. Klapötke, P. C. Schmid, S. Schnell and J. Stierstorfer, J. Mater. Chem. A, 2015, 3, 2658–2668 RSC.
- N. Fischer, T. M. Klapötke, M. Reymann and J. Stierstorfer, Eur. J. Inorg. Chem., 2013, 2167–2180 CrossRef CAS.
- (a) C. Y. Zhang, X. G. Xue, Y. F. Cao, Y. Zhou, H. Z. Li, J. H. Zhou and T. Gao, CrystEngComm, 2013, 15, 6837–6844 RSC; (b) P. Yin, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Chem.–Asian J., 2017, 12, 378–384 CrossRef CAS PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- P. Yin, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2015, 3, 8606–8612 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Rabuck, K. Raghavachari, J. B. Foesman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, A. Lo. G. Liu, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al- Laham, C. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. Wong, C. Gonzalez and J. A. Pople, Gaussian 03 (Revision E.01), Gaussian, Inc, Wallingford CT, 2004 Search PubMed.
- M. Sucéska, EXPLO5, Version 6.01, Brodarski Institute, Zagreb, Croatia, 2013 Search PubMed.
- (a) 13 U. M. Regulations, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, United Nations Publication, New York, 5th edn, 2009 Search PubMed; (b) 13.4.2 Test 3 (a) (ii) BAM Fallhammer, p. 75 Search PubMed; (c) 13.5.1 Test 3 (b) (i) BAM friction apparatus, p. 104, according to UN recommendations on the transport of dangerous goods: impact, insensitive > 40 J, less sensitive ≥ 35 J, sensitive ≥ 4 J, very sensitive ≤ 3 J; friction, insensitive > 360 N, less sensitive 360 N, 80 N < sensitive < 360 N, very sensitive < 80 N, extremely sensitive < 10 N Search PubMed.
- Bruker SAINT (SADABS), v2008/1, Bruker AXS Inc., Madison, Wisconsin, 2008 Search PubMed.
- Bruker SHELXTL, v2008/1, Bruker AXS Inc., Madison, Wisconsin, 2008 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of X-ray crystallography, including additional figures, crystallographic data, and bond lengths and angles data for 1, 1a·H2O, 1b–1c. Details of hydrogen-bonding interactions for 1, 1a·H2O, 1b–1c. CCDC 1916325–1916328. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra09555a |
| ‡ These authors contributed equally to this work, and they did the majority of this work. |
| This journal is © The Royal Society of Chemistry 2020 |