Open Access Article
Open Access ArticleFouling analysis and permeate quality evaluation of mulberry wine in microfiltration process†
Qin-Mei Xiong ab,
Jian Liuab,
Miao Liuc,
Cai-Hong Shenc,
Xue-Chun Yud,
Chong-De Wu
ab,
Jian Liuab,
Miao Liuc,
Cai-Hong Shenc,
Xue-Chun Yud,
Chong-De Wu ab,
Jun Huangab,
Rong-Qing Zhouabe and
Yao Jin*ab
ab,
Jun Huangab,
Rong-Qing Zhouabe and
Yao Jin*ab
aCollege of Biomass Science and Engineering, Sichuan University, Chengdu 610065, China. E-mail: yaojin12@scu.edu.cn; Fax: +86-28-85405237; Tel: +86-138-8219-7633
bKey Laboratory for Leather and Engineering of the Education Ministry, Sichuan University, Chengdu 610065, China
cLu Zhou Lao Jiao Co.,Ltd, Luzhou 646000, China
dLuzhou Pinchuang Technology CO.,LTD, Luzhou 646000, China
eNational Engineering Research Center of Solid-State Manufacturing, Luzhou 646000, China
First published on 2nd January 2020
Abstract
Sterilization and clarification are essential to produce wine of high quality and stability, microfiltration is a serious candidate for both purposes. In this work, microfiltration of fermented mulberry wine was evaluated for the first time. Four different commercial membranes, of two different materials (PES, PVDF) and two different nominal pore sizes (0.22 μm and 0.45 μm) were employed. Pore blocking model was used to identify the fouling mechanism, foulant constituents were revealed by FT-IR spectra. The effect of microfiltration on permeate quality of mulberry wine was also involved. The results indicated that cake formation was the dominant mechanism during steady-state of mulberry wine microfiltration, independently on the membrane property. The fouling layer was mainly composed of protein and polysaccharides, which induced basically reversible overall filtration resistance. Microfiltration delivered a superior clarity, highly polydisperse and light-color mulberry wine with a satisfactory sterilization stability. It preserved the main basic properties and organic acid contents of mulberry wine while resulted in certain loss of volatile compounds, especially esters and alcohols. This work has provided a scientific reference for producing mulberry wine, a modern functional beverage.
1. Introduction
Mulberry wine has been reported with multiple beneficial pharmacological effects thanks to its high content of various bioactive substances, such as organic acids, polyphenols, especially anthocyanins and resveratrol, thus becoming a modern functional beverage of interest.1–3 In the last decades, it has been widely investigated from the perspective of yeast strain,4,5 fermentation/aging process1,6 and polyphenol/volatile compounds.7,8The above-mentioned works have established a basic understanding for the development of mulberry wine production. However, current investigation is far from adequate to sustain a high-efficiency production of mulberry wine. The outcome of high quality mulberry wine after fermentation and aging process is reachable, the gain of a long-term stable high-quality wine is now the challenge, which requires an urgent investigation of the final process before package: sterilization and clarification.
Microfiltration is the widely used process for sterilization and clarification of grape wine and cider.9 Not only it can provide products with superior clarity while keeping most of the flavor and nutrition substances, but also it is able to remove the microorganisms to stop the fermentation and stabilize wine. Microfiltration of grape wine has already been investigated for decades. Membrane fouling of such process has always been the main obstacle of its large-scale application.10 The fouling build-up during wine microfiltration was reported to be mainly governed by the fine colloidal particles, referring to lyophilic macromolecules (polysaccharides), which caused severe irreversible fouling, while large particles (>2 μm) contributed to the cake formation and dominated the overall membrane resistance evolution.11 The adsorption of polyphenolic compounds originating from wine was also showed to impact the microfiltration performance, due to fouling resulting from the interaction between polyphenols and the membrane surface.14
Such works on grape wine have provided a solid reference to explore the process of mulberry wine microfiltration. However, they can not offer direct guiding information for mulberry wine production, after all, different raw fermentation feeds (fruits) exhibited diverged properties which may result in unexpectedly different outcomes. Both grape fruits and mulberry fruits are excellent sources of polyphenols,15 but they are quite different in terms of bioactive compounds and mineral composition,16–18 making the dedicated investigation on mulberry wine microfiltration necessary. Moreover, membrane fouling is a complex outcome of feed characteristics,12,19 membrane properties13,20 and operating conditions,21 which necessitates a particular attention of research work.
Therefore, the aim of this work was to provide a guidance for the process of mulberry wine microfiltration, by analyzing the microfiltration performance with different membranes, then relating to the pore blocking mechanism and foulant identification. This work also explored the effect of microfiltration on the quality of mulberry wine, for the sake of delivering a multi-aspect evaluation of such process. From the best of our knowledge, this is the first time that microfiltration of mulberry wine is being evaluated, which is expected to contribute to the foundation build-up for further development of such process and reference for relevant research.
2. Materials and methods
2.1 Wine preparation and chemicals
Ripe mulberry fruits (Morus notabilis C. K. Schneid) were purchased from local supplier in Sichuan province (China). 60 ppm of SO2 was added into the crushed fruits to inhibit bacteria growth and prevent oxidation. After the adjustment of nitrogenous and carbonate source, two types of commercial yeasts (Zymaflore Alpha, Lalvin D254) were added to initiate fermentation, which then lasted 8 days under 25 °C in a stainless fermenter. When the alcohol content reached 12% v/v and the residual sugar dropped to 2%, the pomace was removed to conduct secondary fermentation for another 10 days under 17 °C. Subsequently, the ferment was filtrated by cheesecloth and then stabilized in the darkness at 2 °C for 15 days. The mulberry wine involved in this study was then sampled after this process, involving two kinds of samples: the immediately sampled one after stabilization referred to samples of natural clarification, the others referred to the permeate of microfiltration.All chemical reagents used in this study were purchased from Sigma-Aldrich (St. Louis, Mo, USA) and Aladdin (Shanghai, China); they were of analytical grade.
2.2 Experimental set-up and procedure
Four sheet membranes with pore sizes of 0.22 μm and 0.45 μm, made of polyethersulfone (PES, Millipore, Co. Ltd., USA) and polyvinylidene fluoride (PVDF, Millipore, Co. Ltd., USA) were used in this study. The specific membrane properties are shown in Table 1. The membrane surface porosity was determined by analyzing the scanning electron microscope (SEM) images with the software Image-Pro Plus (version 6.0, Media Cybernetics, Co. Ltd., America). The hydrophilicity of the membrane was expressed by the contact angle measured by a Video based contact angle measuring device (OCAH200, DataPhysics, Co. Ltd., Germany). Prior to use, all the membranes were soaked in water for 24 hours to remove impurities or additives from the fabrication process.| Material | Module | Filtration area | Pore size (μm) | Porosity (%) | Contact angle (°) | Denote |
|---|---|---|---|---|---|---|
| PES | Flat sheet | 0.0045 m2 | 0.22 | 28.97 ± 2.90 | 44.2 ± 0.3 | PES0.22 |
| PES | 0.45 | 43.75 ± 2.64 | 52.7 ± 0.0 | PES0.45 | ||
| PVDF | 0.22 | 34.82 ± 3.91 | 63.5 ± 0.6 | PVDF0.22 | ||
| PVDF | 0.45 | 42.77 ± 1.17 | 82.1 ± 0.4 | PVDF0.45 |
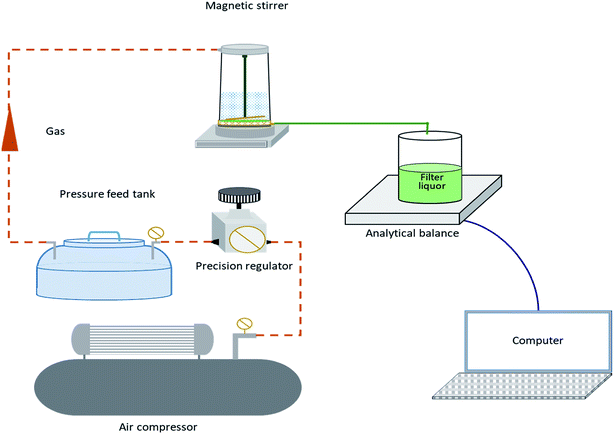
Mulberry wine samples were microfiltered under a transmembrane pressure (ΔP) of 0.06 MPa at ambient temperature (25 ± 1 °C), with the four above-mentioned flat membranes by a commercial stirred (300 rpm) dean-end filtration cell (Millipore, Co. Ltd., USA). Preliminary experiments were conducted to determine the optimal operating conditions mentioned above. The pressure was applied via purified compressed air and was monitored by pressure gauges. The permeate was monitored by a reservoir placed on a digital balance with an accuracy of 0.01 g which was connected to a computer by measuring the mass variation in a reservoir vessel every one minute Fig. 1 illustrates a simplified scheme of the dead-end microfiltration set-up.
At the beginning and at the end of each microfiltration run, permeate flux of distilled water was measured at ΔP = 0.06 MPa, in order to monitor the change of water permeability of membrane after mulberry wine microfiltration.
2.3 Models of membrane fouling analysis
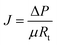 | (1) |
| Rt = Rm + Rf = Rm + Rc + Rcp + Rirrf | (2) |
Rm and Rt can be calculated by the following formula (eqn (3) and (4)):
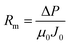 | (3) |
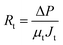 | (4) |
After the microfiltration, the remaining sample inside the filtration cell was gently poured out, then pure water was added to refill the cell, the steady-state permeate flux was measured again at this stage, denoted as J1. Afterwards, the filtration cell and membrane were finally washed with plenty of water, a final water flux was measured, denoted as J2. With the help of J1 and J2, Rc, Rcp and Rirrf can be estimated by the following formula:
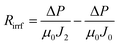 | (5) |
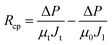 | (6) |
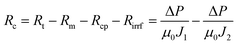 | (7) |
Complete pore blocking:
| j = e−αbt | (8) |
Internal pore constriction blocking:
| j = (1 + 2αit)−2 | (9) |
Cake filtration:
| j = (1 + 2αct)−0.5 | (10) |
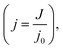 t indicates the filtration time (s), j0 is the initial flux (L m−2 h−1). αb is the system parameter relating to complete pore blocking (s−1), αi is the system parameter relating to internal pore constriction blocking (s−1), αc is the system parameter relating to cake filtration (s−1).
t indicates the filtration time (s), j0 is the initial flux (L m−2 h−1). αb is the system parameter relating to complete pore blocking (s−1), αi is the system parameter relating to internal pore constriction blocking (s−1), αc is the system parameter relating to cake filtration (s−1).
2.4 Analytical methods
A single sample of 20 μL was then injected into an Agilent 1260 HPLC system equipped with an Alltech OA-1000 organic acid column (300 × 78 mm) maintained at 75 °C with UV detector (210 nm). All samples were measured in triplicate.
GC-MS apparatus (Trace GC Ultra gas chromatograph-DSQ II mass spectrometer, Thermo Electron Corporation, Waltham, America) equipped with an HP-INNOWAX capillary column (30.0 m × 0.25 mm × 0.25 μm, Agilent Technology, Santa, USA) was used to separate and detect the volatile components of the mulberry wine samples. The injector temperature was set to 250 °C, high purity helium gas (99.999%) with a flow rate of 1.00 mL min−1 was used as the carrier gas. The split mode was used and the ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then oven temperature was programmed as follows: the initial temperature was 40 °C for 5 min, then ramped at 4 °C min−1 to 100 °C and then 6 °C min−1 to 220 °C for 8 min. The mass spectrum was operated in the positive ion electron impact ionization (EI+) mode at 70 eV in a range of 35–400 amu. The temperature of the ion source and transfer line were set at 230 °C and 250 °C, respectively.
1. Then oven temperature was programmed as follows: the initial temperature was 40 °C for 5 min, then ramped at 4 °C min−1 to 100 °C and then 6 °C min−1 to 220 °C for 8 min. The mass spectrum was operated in the positive ion electron impact ionization (EI+) mode at 70 eV in a range of 35–400 amu. The temperature of the ion source and transfer line were set at 230 °C and 250 °C, respectively.
The identification and retention index of each volatile were respectively obtained by comparing their mass spectrum with the NIST05 library database (Finnigan Co. Ltd., California, USA). The relative content of each volatile was then determined by the ratio between the peak area of the specific compound and that of the internal standard. All samples were analyzed in triplicate and the results were noted as the mean value ± relative standard deviation (RSD).
3. Results and discussion
3.1 Microfiltration performance
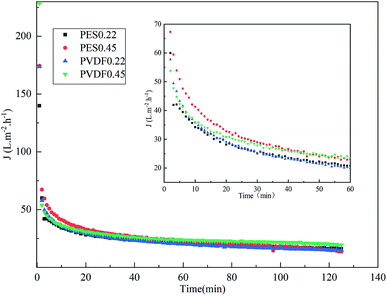
Fig. 2 shows the evolution of permeate flux over time during the whole microfiltration process for different membranes. Basically, a quick and dramatic decline occurred during microfiltration, with whichever membrane. The membranes with bigger nominal pore size (0.45 μm) showed higher permeate flux at the early stage of process, which then progressively declined into the same flux level to the membranes with smaller nominal pore size (0.2 μm). Among the tested membranes, PVDF0.45 showed the highest steady-state permeate flux.Table 2 decomposes the steady-state fouling resistance for each membrane at ΔP = 0.06 MPa according to resistance-in-series model, incorporated in eqn (3)–(7). The total resistances (Rt) after microfiltration were quite comparable among the tested membranes, around 8 × 1012 m−1, which were mainly composed of reversible fouling (Rc and Rcp) accounting for more than 98% of the total resistance, among which the cake resistance (Rc) was higher than concentration polarization resistance (Rcp). It indicates that the cake formation may be the main mechanism during microfiltration of mulberry wine. It is also noticeable that the irreversible fouling resistance (Rirrf) was more severe in the case of 0.22 μm than 0.45 μm, independently on the membrane materials.
| Membranes | Microfiltration resistance (m−1) | ||||
|---|---|---|---|---|---|
| Rt × 1012 | Rm × 1010 | Rirrf × 109 | Rc × 1012 | Rcp × 1012 | |
| PES0.22 | 7.59 | 5.63 | 7.07 | 6.26 | 1.32 |
| PES0.45 | 8.17 | 5.44 | 4.73 | 4.95 | 3.22 |
| PVDF0.22 | 8.03 | 9.09 | 7.51 | 6.81 | 4.33 |
| PVDF0.45 | 8.59 | 5.44 | 1.55 | 6.79 | 1.78 |
3.2 Pore blocking mechanism
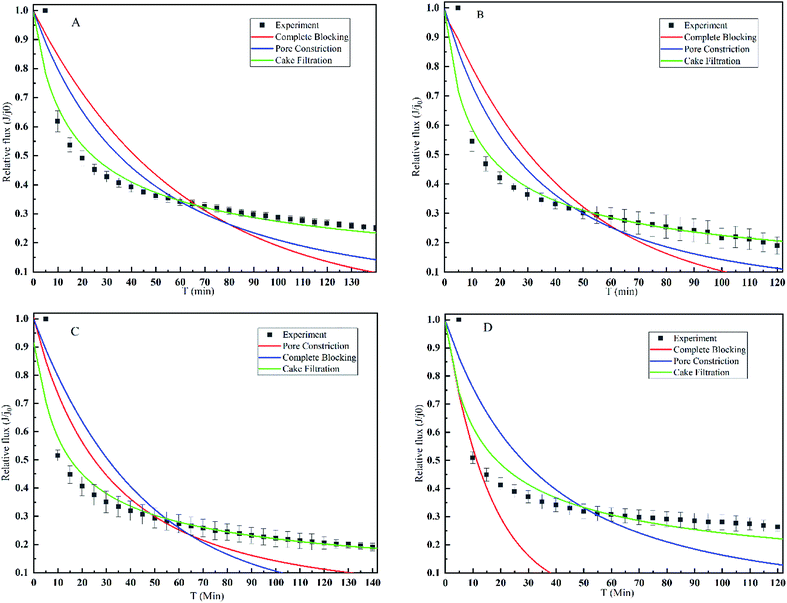
In order to further explore the fouling mechanism of mulberry wine microfiltration, pore blocking model was employed. Fig. 3 illustrates the fitting results of different pore blocking model derivations on the filtration data according to eqn (8)–(10). The model rate constants and regression coefficients are summarized in Table 3. All the experiments were carried out at the same operating conditions. Regarding the regression coefficients R and comparison between the fitted lines and the experimental data shown in and Table 3, cake filtration model fitted well the experimental data with no matter which membrane. Otherwise, neither complete blocking nor pore constriction could describe the fouling mechanism of mulberry wine microfiltration since the regression coefficients were too low for both. It is also noticeable that the filtration data of early stage did not perfectly fit into the three applied models, implying that the mechanism at this stage of microfiltration needed further dedicated investigation.| Membrane (nm) | Blocking | Constriction | Cake | |||
|---|---|---|---|---|---|---|
| αb (min−1) | R | αp (min−1) | R | αc (min−1) | R | |
| PES0.22 | 0.01663 | 0.5039 | 0.0059 | 0.7717 | 0.06165 | 0.9511 |
| PES0.45 | 0.02316 | 0.635 | 0.00836 | 0.8319 | 0.09215 | 0.9304 |
| PVDF0.22 | 0.02249 | 0.4764 | 0.00826 | 0.7772 | 0.098 | 0.9281 |
| PVDF.45 | 0.06039 | — | 0.00735 | 0.582 | 0.07995 | 0.8877 |
Such results confirmed the suggestion of resistance-in-series model, revealed that the dominant fouling mechanism during microfiltration of mulberry wine in steady-state was indeed cake deposition, which was barely impacted by the membrane property.
3.3 Foulant identification
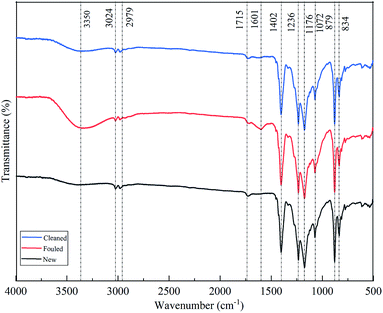
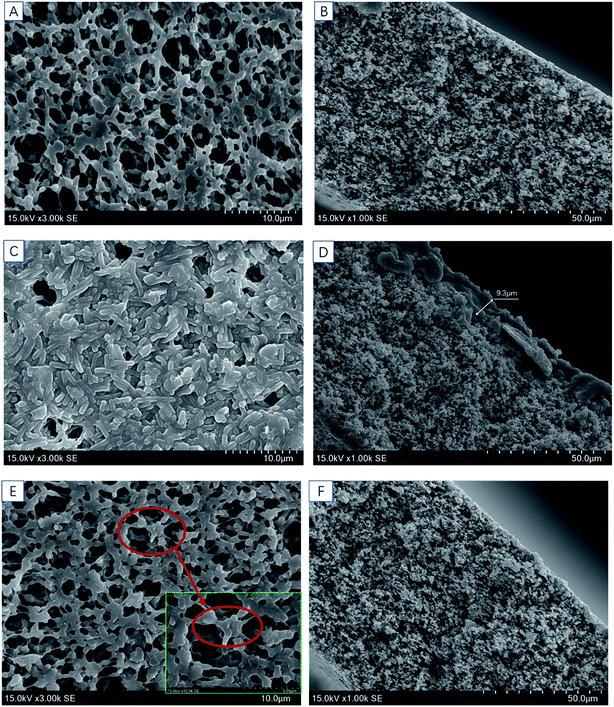
ATR-FTIR and SEM were employed to identify the foulants on the membrane. Fig. 4 shows the FTIR spectra and corresponding band vibration of the pristine, fouled and cleaned membranes of PVDF0.45. As shown in this figure, all the membranes showed the same spectral characteristics of a PVDF at 834 cm−1, 879 cm−1, 1072 cm−1, 1176 cm−1, 1236 cm−1, 1402 cm−1, which correspond to CH2 rocking, C–C asymmetric stretching, CF2 symmetric stretching, CF out of plane deformation, and CH2 wagging, respectively.32 This signifies that the intrinsic characteristics of membrane did not alter after fouling. The main difference between the fouled membrane and the others (new and cleaned) was the peaks of 1601 cm−1 and 3350 cm−1, corresponding to the characteristic peaks of peptide bond in proteins and polysaccharides according to previous reports.33 Such results indicate that the organic foulant of protein and polysaccharides was the major foulant compounds on/in the membrane.The microstructure of the membrane was evidenced by SEM. Fig. 6a and b shows the microstructure of the pristine membrane, with open membrane surface and no dirt. After microfiltration of mulberry wine, layered deposits appeared on the membrane surface (Fig. 5c) so that the membrane pores were sheltered. The cleaned membrane (Fig. 5e and f) again showed similar morphology to the pristine one, indicating an effective cleaning. The cross-section morphology of membranes then revealed the gross thickness of this deposit. As can be seen from Fig. 5b, d and f, the morphologies of the pristine and the cleaned membrane show a visualized coincidence indicating a non-deposition structure, while the fouled one was covered with a deposit of around 10 μm.
Combined the results of ATR-FTIR and SEM, one can claim that a deposit of 10 μm (dry), consisting of protein and polysaccharides, would form on the membrane surface during microfiltration of mulberry wine, and such deposit was supposed to be reversible, since pure water cleaning already showed a good efficiency to remove it.
3.4 Permeate quality
In this section, effect of microfiltration on the permeate quality of mulberry wine samples was investigated, focusing on the physicochemical properties, organic acids, volatile compounds and storing stability. A sample subjected to natural clarification was also involved in this part to provide comparative reference to the microfiltrated samples.| Parameters | Natural clarification | PES0.22 | PES0.45 | PVDF0.22 | PVDF0.45 |
|---|---|---|---|---|---|
| a Mean values in the same row with different superscripted letters indicate that they are significantly different at p < 0.05. ANOVA analysis was applied. | |||||
| Alcoholic strength (V/V%) | 12.8 | 12.4 | 12.8 | 12.5 | 12.7 |
| Total sugar (g/100 mL) | 2.95 ± 0.01b | 2.98 ± 0.00a | 2.92 ± 0.10a | 2.96 ± 0.02a | 2.99 ± 0.03a |
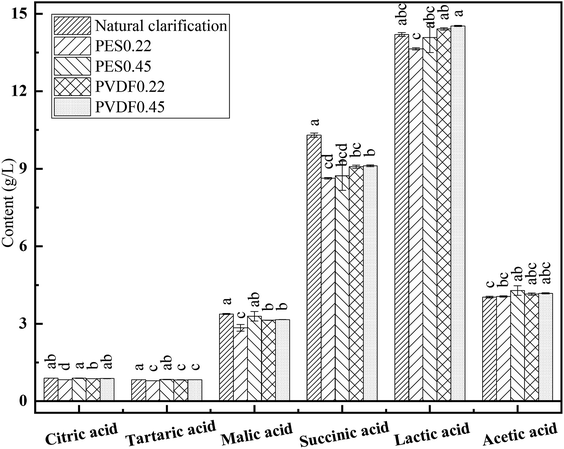
| Total acid (g L−1) | 10.86 ± 0.05a | 10.59 ± 0.06b | 10.68 ± 0.06b | 10.22 ± 0.01c | 10.71 ± 0.11b |
| pH | 4.87 ± 0.01a | 4.83 ± 0.01b | 4.83 ± 0.01b | 4.83 ± 0.01b | 4.87 ± 0.01a |
| TSS (°Brix) | 11.93 ± 0.06a | 11.90 ± 0.00a | 11.80 ± 0.00b | 11.90 ± 0.00a | 11.80 ± 0.00b |
| Turbidity (NTU) | 372.00 ± 2.65a | 1.31 ± 0.06b | 1.41 ± 0.02b | 1.26 ± 0.03b | 1.30 ± 0.05b |
| Chroma | 3.185 ± 0.046a | 2.553 ± 0.026c | 2.644 ± 0.038b | 2.564 ± 0.030c | 2.594 ± 0.030bc |
| Total phenol (g L−1) | 6.21 ± 0.02a | 6.02 ± 0.02d | 6.07 ± 0.02bc | 6.03 ± 0.03 cd | 6.08 ± 0.03b |
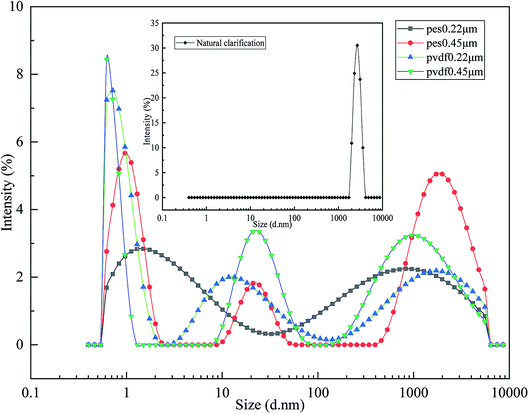
| Particle mean size (nm) | 2350 ± 152.88a | 15.02 ± 2.91b | 54.81 ± 14.90b | 17.58 ± 0.98b | 29.20 ± 1.68b |
| PDI | 0.26 ± 0.06c | 0.59 ± 0.03b | 0.92 ± 0.13a | 0.60 ± 0.04b | 0.83 ± 0.07a |
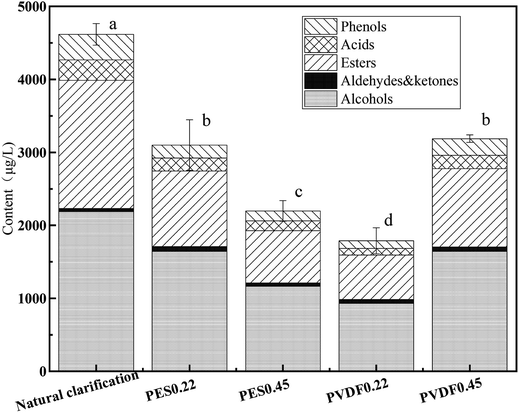
Fig. 8 illustrates the content and proportion of 6 volatile groups in different mulberry samples. Esters and alcohols were the dominant volatile compounds in mulberry wine samples, accounting for more than 85% of the total volatile compounds. The most abundant volatile compounds found in these mulberry wine samples were: 3-methyl-1-butanol, phenethyl alcohol, butanedioic acid diethyl ester and eugenol, with concentration superior to 300 μg L−1 (Table S1†). These compounds contributed a great deal to the characteristic aroma of mulberry wine, consistent with the previous reports in the literature.5,36 After microfiltration, the content of total volatile compounds of mulberry wine samples declined to certain extent, especially for esters, alcohols and phenols. Among the 4 tested membranes, PVDF0.45 performed the best in terms of volatile compound preservation, PES0.22 also delivered a fairly good performance. Interestingly, the four tested membranes, of different materials and nominal pore sizes, did not show a convergent trend: the volatile compound preservation performance of them followed a scattered order (PVDF0.45 > PES0.22 > PES0.45 > PVDF0.22), the retention ratio of volatile compounds by membranes seemed to neither depend on the membrane material type nor the nominal pore size, but the combination of both.
| Parameters | Date | Natural clarification | PES0.22 | PES0.45 | PVDF0.22 | PVDF0.45 |
|---|---|---|---|---|---|---|
| Total bacteria (CFU mL−1) | d0 | 140 | <1 | <1 | <1 | <1 |
| d10 | 1.68 × 105 | <1 | <1 | <1 | <1 | |
| d20 | 3.70 × 105 | <1 | <1 | <1 | <1 | |
| d30 | 6.62 × 105 | <1 | <1 | <1 | <1 | |
| d40 | 1.2 × 106 | <1 | <1 | <1 | <1 | |
| Total yeast (CFU mL−1) | d0 | 2000 | <1 | <1 | <1 | <1 |
4. Conclusion
In this work, four different widely-used commercial membranes, of two different materials (PES, PVDF) and two different nominal pore sizes (0.22 μm and 0.45 μm) were employed to perform microfiltration of mulberry wine. Cake formation, mainly consisting of protein and polysaccharides, was the dominant mechanism during steady state of such process, independently on the membrane property. Fouling in this process was mainly reversible. Microfiltration was able to deliver a superior clarity, highly polydisperse and light-color mulberry wine with a satisfactory sterilization stability and well preserve the main basic properties and organic acid contents while induced certain loss of volatile compounds, especially esters and alcohols. Pore size indeed influenced the particle mean size and polydispersity of permeate, while the loss of volatile compounds depended on a complex combination of membrane material and pore size. This is the first time that the process of mulberry wine microfiltration has been systematically evaluated from the aspects of both fouling mechanism and permeate quality, providing guidance for the further development of mulberry wine production.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was sponsored by the Fundamental Research Funds for the Central Universities of China (Grant number: YJ201835), Sichuan university-Luzhou cooperation project (Grant number: 2017CDLZ-S18) and National Science Foundation of China (Grant number: 21978175).References
- W. Tchabo, Y. Ma, E. Kwaw, H. Zhang, X. Li and N. A. Afoakwah, Food Bioprocess Technol., 2017, 10, 1210–1223 CrossRef CAS.
- Y. You, X. Yuan, H. J. Lee, W. Huang, W. Jin and J. Zhan, Food Funct., 2015, 6, 401–408 RSC.
- W. Tchabo, Y. Ma, E. Kwaw, H. Zhang, L. Xiao and M. T. Apaliya, Food Chem., 2018, 239, 470–477 CrossRef CAS.
- S. Liu, C. Wu, G. Fan, T. Li, R. Ying and Y. Miao, J. Food Biochem., 2017, 41, e12409 CrossRef.
- X. Ouyang, B. Zhu, R. Liu, Q. Gao, G. Lin, J. Wu, Z. Hu and B. Zhang, J. Food Process. Preserv., 2018, 42, e13432 CrossRef.
- L. Wang, X. Sun, F. Li, D. Yu, X. Liu, W. Huang and J. Zhan, J. Funct. Foods, 2015, 18, 254–265 CrossRef CAS.
- S. Liu, E. Liu, B. Zhu, B. Chai, R. Liu, Q. Gao and B. Zhang, J. Inst. Brew., 2018, 124, 45–56 CrossRef CAS.
- W. Tchabo, Y. Ma, E. Kwaw, H. Zhang, L. Xiao and H. E. Tahir, Food Chem., 2017, 232, 89–97 CrossRef CAS PubMed.
- Y. El Rayess, C. Albasi, P. Bacchin, P. Taillandier, J. Raynal, M. Mietton-Peuchot and A. Devatine, J. Membr. Sci., 2011, 382, 1–19 CrossRef CAS.
- Y. E. Rayess, Y. Manon, N. Jitariouk, C. Albasi, M. M. Peuchot, A. Devatine and L. Fillaudeau, J. Membr. Sci., 2016, 513, 47–57 CrossRef.
- A. Vernhet, D. Cartalade and M. Moutounet, J. Membr. Sci., 2003, 211, 357–370 CrossRef CAS.
- W. Zhang and F. Jiang, Water Res., 2019, 157, 445–453 CrossRef CAS PubMed.
- W. Chen, J. Mo, X. Du, Z. Zhang and W. Zhang, Water Res., 2019, 151, 243–251 CrossRef CAS PubMed.
- M. Ulbricht, W. Ansorge, I. Danielzik, M. König and O. Schuster, Sep. Purif. Technol., 2009, 68, 335–342 CrossRef CAS.
- I. Khalifa, W. Zhu, K. Li and C. Li, J. Funct. Foods, 2018, 40, 28–43 CrossRef CAS.
- S. Ercisli and E. Orhan, Food Chem., 2007, 103, 1380–1384 CrossRef CAS.
- Q. Yuan and L. Zhao, J. Agric. Food Chem., 2017, 65, 10383–10394 CrossRef CAS.
- T. M. Gomes, I. M. Toaldo, I. C. da S. Haas, V. M. Burin, V. Caliari, A. S. Luna, J. S. de Gois and M. T. Bordignon-Luiz, J. Funct. Foods, 2019, 52, 699–708 CrossRef CAS.
- Y. Jin, N. Hengl, S. Baup, G. Maitrejean and F. Pignon, J. Membr. Sci., 2017, 528, 34–45 CrossRef CAS.
- J. Teng, L. Shen, Y. He, B.-Q. Liao, G. Wu and H. Lin, Chemosphere, 2018, 210, 769–778 CrossRef CAS PubMed.
- Y. Jin, N. Hengl, S. Baup, F. Pignon, N. Gondrexon, M. Sztucki, G. Gésan-Guiziou, A. Magnin, M. Abyan, M. Karrouch and D. Blésès, J. Membr. Sci., 2014, 470, 205–218 CrossRef CAS.
- H. Guo, J. Huang, R. Zhou, C. Wu and Y. Jin, RSC Adv., 2019, 9, 2928–2940 RSC.
- A. L. Lim and R. Bai, J. Membr. Sci., 2003, 216, 279–290 CrossRef CAS.
- M. Li, Y. Zhao, S. Zhou and W. Xing, Desalination, 2010, 256, 166–173 CrossRef CAS.
- M. Li, Y. Zhao, S. Zhou, W. Xing and F.-S. Wong, J. Membr. Sci., 2007, 299, 122–129 CrossRef CAS.
- R. Sondhi, Y. S. Lin and F. Alvarez, J. Membr. Sci., 2000, 174, 111–122 CrossRef CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, in Oxidants and Antioxidants, Pt A, ed. L. Packer, Elsevier Academic Press Inc, San Diego, 1999, vol. 299, pp. 152–178 Search PubMed.
- Z. M. Sharif, M. S. Othman and N. J. Jalil, AIP Conf. Proc., 2018, 020082 CrossRef.
- L. Zhang, J. Huang, R. Zhou and C. Wu, Int. J. Food Microbiol., 2017, 255, 42–50 CrossRef CAS PubMed.
- X. Ding, C. Wu, J. Huang and R. Zhou, LWT–Food Sci. Technol., 2016, 66, 124–133 CrossRef CAS.
- Y. Jin, D. Li, M. Ai, Q. Tang, J. Huang, X. Ding, C. Wu and R. Zhou, Food Res. Int., 2019, 121, 422–432 CrossRef CAS.
- P. Nallasamy and S. Mohan, Indian J. Pure Appl. Phys., 2005, 43, 821–827 CAS.
- F. Zhao, H. Chu, X. Tan, L. Yang, Y. Su, X. Zhou, J. Zhao and Y. Zhang, J. Membr. Sci., 2016, 517, 30–38 CrossRef CAS.
- V. Ivanova-Petropulos, Z. Nace Va, V. Sand Or, L. Maksz In, L. Na Gy, B. Berki Cs, T. Stafilov and F. Kilar, Electrophoresis, 2018, 39, 1597–1605 CrossRef CAS PubMed.
- N. Markkinen, O. Laaksonen, R. Nahku, R. Kuldjärv and B. Yang, Food Chem., 2019, 286, 204–215 CrossRef CAS PubMed.
- C. Juan, K. Jianquan, T. Junni, C. Zijian and L. Ji, J. Food Sci., 2012, 77, C430–C436 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09034g |
| This journal is © The Royal Society of Chemistry 2020 |