 Open Access Article
Open Access ArticleNanozyme-based catalytic theranostics
Yanan Zhanga,
Yiliang Jina,
Haixia Cuib,
Xiyun Yanac and
Kelong Fan *ac
*ac
aCAS Engineering Laboratory for Nanozyme, Key Laboratory of Protein and Peptide Pharmaceutical, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China. E-mail: fankelong@ibp.ac.cn
bDepartment of Clinical Laboratory, Yidu Central Hospital of Weifang, Weifang 262500, Shandong, China
cJoint Laboratory of Nanozymes in Zhengzhou University, Academy of Medical Sciences, Zhengzhou University, 40 Daxue Road, Zhengzhou 450052, China
First published on 23rd December 2019
Abstract
Nanozymes, a type of nanomaterial with intrinsic enzyme-like activities, have emerged as a promising tool for disease theranostics. As a type of artificial enzyme mimic, nanozymes can overcome the shortcomings of natural enzymes, including high cost, low stability, and difficulty in storage when they are used in disease diagnosis. Moreover, the multi-enzymatic activity of nanozymes can regulate the level of reactive oxygen species (ROS) in various cells. For example, superoxide dismutase (SOD) and catalase (CAT) activity can be used to scavenge ROS, and peroxidase (POD) and oxidase (OXD) activity can be used to generate ROS. In this review, we summarize recent progress on the strategies and applications of nanozyme-based disease theranostics. In addition, we address the opportunities and challenges of nanozyme-based catalytic theranostics in the near future.
A nanozyme is a type of nanomaterial (1–100 nm) with enzyme-like activities.1,2 It can catalyze the reaction of enzyme substrates under physiological conditions, and it has similar catalytic efficiency and enzymatic abilities to natural enzymes. Our previous work found that Fe3O4 nanoparticles (NPs) possess an intrinsic peroxidase (POD)-like activity.3 Since then, numerous nanomaterials have been discovered to have POD-, catalase (CAT)-, superoxide dismutase (SOD)-, or oxidase (OXD)-like catalytic activities.4 A nanozyme may have more than one type of catalytic activity.5 Nowadays, more than 540 nanozymes from 49 elements have been reported from 350 laboratories in 30 countries.6,7 Among these, iron oxide nanoparticles,8 CeO2,9 graphene oxide,10 carbon nanozymes11 and gold nanoparticles12 are widely studied and applied.
Nanozymes can simulate the catalytic processes of natural enzymes and regulate the redox level of cells, especially on reactive oxygen species (ROS). ROS are intermediate products which emerge in the process of oxygen metabolism, mainly including superoxide anion (O2˙−), hydroxyl radical (·OH), and hydrogen peroxide (H2O2).13 An abnormal rise in ROS level will destroy the homeostasis of redox in vivo and cause oxidative stress. Nanozymes typically exhibit multiple enzymatic activities. On the one hand, the catalase and superoxide dismutase activity of nanozymes are mainly used to regulate the intracellular ROS level, which plays an important role in protecting cells. On the other hand, the oxidase and peroxidase activity of nanozymes induce ROS production and promote apoptosis, such as in cancer cells.
With advantages such as high catalytic efficiency, high stability, biosafety, low cost and easy preparation,14 nanozymes have been widely used in industrial, medical, and biological fields and in environmental remediation.2,15,16 Currently, a variety of nanozyme-based biomedical applications have been extensively explored, including biosensors,17 in vitro texts,18 and antimicrobial19 and disease treatments, such as cancer therapy, bone marrow therapy and wound healing.20 Here, we summarize the biomedical applications of nanozymes in vivo, as well as addressing the opportunities and challenges of nanozyme-based catalytic disease theranostics in the near future.
1 Nanozyme-based disease diagnosis
As bifunctional or multifunctional molecules,21 nanozymes possess not only enzyme-like catalytic activity, but also the physical and chemical properties of nanomaterials, such as magnetic and photothermal properties. Compared with natural enzymes, nanozymes are favored with high stability, easy modification, simple preparation and low cost. These properties enable nanozymes to be applied in disease diagnosis. In terms of the diagnosis of disease, the peroxidase-like activity of nanozymes is extensively used, followed by their oxidase-like activity and catalase-like activity.1.1 Nanozymes for cancer diagnosis
Cancers are commonly treated by surgery, chemotherapy, and radiotherapy. Early diagnosis of cancer makes it possible to treat primary tumors effectively with local treatments such as surgery and radiotherapy. Early detection is primarily dependent on blood biomarkers. However, the shedding rate of most biomarkers from tumors is extremely low, they are greatly diluted after blood circulation, and the lack of specificity of biomarkers hinders the accuracy and sensitivity of early detection. The appearance of nanozymes provides new ideas and methods for tumor diagnosis by detecting tumor cells and tissues.Nanozymes can be used to detect cancer cells. Zhang et al. proposed the in situ growth of porous platinum nanoparticles on graphene oxide (PtNPs/GO) (Fig. 1A).22 PtNPs/GO has peroxidase activity and can catalyze the reaction of peroxides in the presence of hydrogen peroxide and cause the color reaction of TMB. Then PtNPs/GO was modified with folic acid to specifically target tumor cells with high folic acid receptor expression. FA-PtNPs/GO was employed as a signal transducer to develop a colorimetric assay for the direct detection of cancer cells. PtNPs/GO can detect 125 cancer cells by observation with the naked eye. Moreover, Wang et al. use graphitic carbon nitride nanosheets (g-C3N4 NSs) to detect breast cancer cells.23 The adsorption of ssDNA on graphite carbonitride nanosheets (g-C3N4 NSs) can improve the catalytic activity of the nanosheets (Fig. 1B). The maximum reaction rate of H2O2 was at least 4 times faster than that of unmodified NSs in TMB oxidation mediated by an ssDNA-NSs hybrid. The high catalytic activity of the ssDNA-NSs hybrid allows for the sensitive colorimetric detection of exosomes when an appropriate target for the exosome surface marker CD63 is used in the construction of the hybrid. The sensor identified a differential expression of CD63 in exosomes from breast cancer cell line (McF-7) and control cell line (McF-10a).
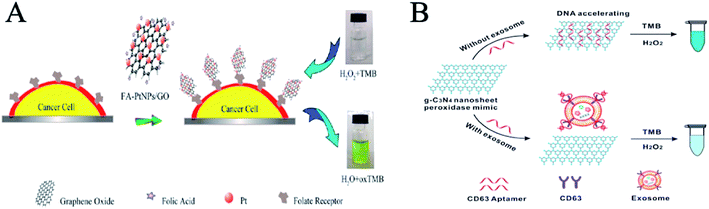 | ||
| Fig. 1 Nanozymes for cancer cell detection. (A) Schematic representation of colorimetric direction of cancer cells by using folic acid functionalized PtNPs/GO (reprinted from ref. 22 with permission from Anal. Chem.). (B) Illustration of DNA aptamer accelerating the intrinsic peroxidase-like activity of g-C3N4 NSs for the detection of exosomes (reprinted from ref. 23 with permission from Anal. Chem.). | ||
The diagnosis of tumor tissue is crucial in cancer detection. Fan et al. found that magnetic ferritin nanozymes (M-HFn) can be used to target and visualize tumor tissues without any targeted ligands or contrast agents.24 Ferritin is an iron storage protein that plays a key role in iron homeostasis and the anti-oxidation of cells.25 Iron oxide nanozymes are encapsulated in a shell of recombinant human heavy chain ferritin (HFn), which binds to tumor cells that overexpress transferrin receptor 1 (TfR1). Iron oxide nanozyme catalyzes the oxidation of peroxidase substrates in the presence of hydrogen peroxide to produce a chromogenic reaction for the observation of tumor tissues. This diagnostic method detects tumor tissue with 98% sensitivity and 95% specificity (Fig. 2A). In addition, the ferritin nanozyme diagnostic technique is simple, rapid, and economic. This method simplifies the conventional immunohistochemical experiment for tumor detection and shortens the testing time from 4 hours to 1 hour, which greatly improves the efficiency of clinical pathological diagnosis.
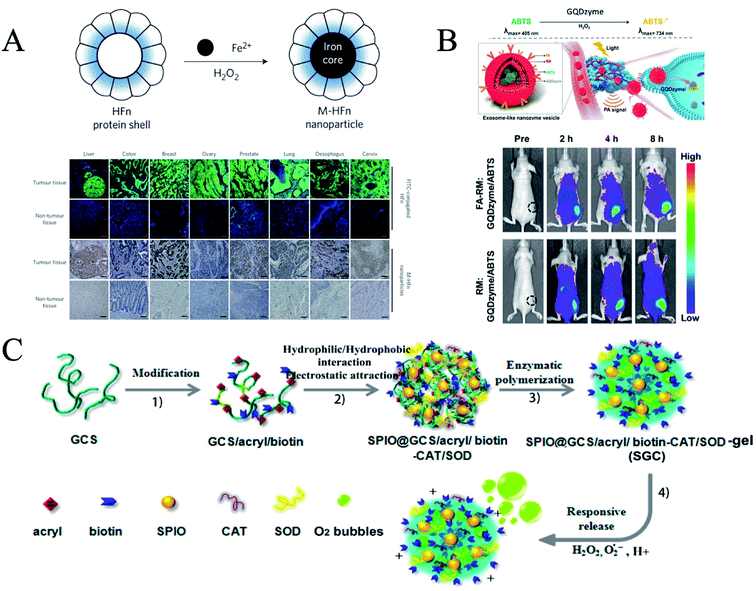 | ||
| Fig. 2 Nanozymes for tumor diagnosis. (A) Cancer diagnosis in clinical specimens using M-HFn nanoparticles (reprinted from ref. 25 with permission from Nat. Nanotechnol.). (B) Schematic illustration of exosome-like nanozyme vesicles for catalytic photoacoustic imaging of NPC tumors (reprinted from ref. 26 with permission from Nano Lett.). (C) Schematic illustration of the SGC (SPIO@GCS/acryl/biotin-CAT/SOD-gel) nanogel (reprinted from ref. 28 with permission from ACS Nano). | ||
Photoacoustic imaging (PAI) can provide high-resolution tissue imaging, and is a very promising imaging method. The peroxidase activity of graphene quantum dot nanozymes (GQDzymes) can effectively convert 2,2′-azino-bis(3-ethylbenzothiazolin-6-sulfonic acid) (ABTS) into its oxidized form in the presence of H2O2. Oxidized ABTS exhibits a strong near infrared (NIR) absorbance. Ding et al. developed a folate-modified erythrocyte membrane coated GQDzyme/ABTS as a nanozyme vesicle, which can target tumor tissues (Fig. 2B).26 The acidic tumor microenvironment provides H2O2 for the production of PAI agents to diagnose nasopharyngeal carcinoma.
Ultrasmall gold nanoclusters (AuNCs) have been used as a sensitive probe for in vivo imaging due to their excellent tumor enrichment and kidney clearance. Loynachan et al. constructed a multifunctional protease nanosensor using AuNCs with peroxidase-like activity.27 It can respond to the disease microenvironment to generate colorimetric signal readings and monitor disease status in less than an hour. They monitored the catalytic activity of AuNCs in the collected urine of a mouse model of colorectal cancer in which tumor-bearing mice showed a 13-fold increase in colorimetric signal compared to healthy mice. The nanosensors were eliminated completely through hepatic and renal excretion within four weeks of injection with no evidence of toxicity. This method can be used to visually observe the color changes caused by the catalytic activity of urine nanoclusters to TMB, determining whether the tissue is cancerous.
The use of magnetic resonance imaging (MRI) in tumor diagnosis is challenged by its low sensitivity. Xi et al. introduced functional magnetic resonance imaging of inorganic components (iron and manganese) into a nanogel through self-assembly and in situ reduction processes.28 A multifunctional hybrid nanogel probe with dual enzymes (SPIO@GCS/acryl/biotin-CAT/SOD-gel, or SGC) (Fig. 2C) was developed for dual-mode pathologic response ultrasound (US) imaging and enhanced T-2 weighted magnetic resonance imaging. The probe consists of functionalized superparamagnetic iron oxide particles, a dual enzyme species (catalase and superoxide dismutase) and a polysaccharide cationic polymer glycol chitosan gel. The dual-mode US/MR imaging capabilities of the mixed nanocrystals for responsive US imaging and enhanced T-2 weighted MR imaging have been evaluated in vitro and in vivo. The multifunctional hybrid probe with dual enzymes can achieve dual-mode imaging with enhanced tumor region responsiveness, which can greatly improve the sensitivity and efficiency of tumor imaging. In addition, Xi et al. decorated Pd nanoparticles (NPs) with double shell structured N-doped graphene quantum dots (NGQDs)@n-doped carbon (NC) HNS. NGQD ACTS, as a dual signal amplifying nanometer probe, was used as a high-efficiency electrocatalyst in detecting cancer biomarkers.29
1.2 Nanozymes for metabolic disease diagnosis
Metabolic diseases are diseases caused by metabolic disorders and vigorous metabolism, including diabetes, diabetic ketoacidosis and vitamin D deficiency.Diabetes is a metabolic disease characterized by hyperglycemia. Horseradish peroxidase (HRP) has been widely used in the manufacture of glucose oxidase products that are used as sensors to detect diabetes. Qu et al. found that carboxyl-modified GO (GO-COOH) had peroxidase-like activity and could catalyze the reaction of peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB) in the presence of H2O2 to produce a blue reaction.10 This method can be applied to measure the amount of glucose in the blood.
Yao et al. designed a new colorimetric and ratiometric fluorescence detection method for uric acid (UA).30 Yb3+, Er3+ and Tm3+ co-doped NaYF4 nanoparticles (UCNPs) were synthesized which emit upconversion fluorescence with four typical emission peaks centered at 490 nm, 557 nm, 670 nm and 705 nm under 980 nm near-infrared (NIR) irradiation. ZnFe2O4 magnetic nanoparticles (MNPs) with excellent peroxidase-like activity were prepared and used to catalyze the coupling of N-ethyl-N-(3-sulfopropyl)-3-methylaniline sodium salt (TOPS) and 4-amino-antipyrine (4-AAP) in the presence of H2O2 to form purple products with a characteristic absorption peak located at 550 nm. This method has been successfully applied in the analysis of UA in human serum.
1.3 Nanozymes for the other disease diagnosis
Nanozymes can also be employed to diagnose infectious diseases, such as viruses and bacteria. Li et al. found that Hg2+ could selectively and sensitively stimulate the oxidase-like activity of BSA-Ag NCs, then realize the sensitive colorimetry of Hg2+.31 The oxidase-like activity of Hg2+ stimulated BSA-Ag NCs is attributed to Hg2+ binding to the surface of BSA-Au NCs, changing the surface characteristics and promoting the production of superoxide anions for TMB oxidation. By extracting the DNA of human immunodeficiency virus HIV-1, a fast and simple colorimetric method was used to detect HIV. The system can detect 20 nmol L−1 of HIV DNA fragment HIV-1, realizing the diagnosis of infectious diseases. Moreover, nanozyme strips can be used to detect Ebola; in this technology, the colloidal gold in a traditional test strip is replaced by an Fe3O4 magnetic nanoparticle (MNP) nanozyme (Fig. 3), which is 100-fold more sensitive than the standard strip method.32 This technology can not only quickly detect viruses such as Ebola, but also can be used to detect viruses and toxins at customs ports by switching the types of antibodies on the probes.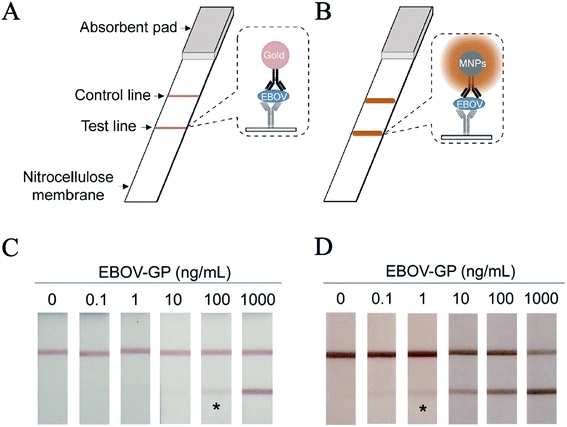 | ||
| Fig. 3 Nanozyme-strip for virus detection. (A) Standard colloidal gold strip. (B) Nanozyme-strip employing MNPs in place of colloidal gold to form a novel nanozyme probe. (C) Standard colloidal gold strip for EBOV-GP detection. (D) Nanozyme-strip for EBOV-GP detection (reprinted from ref. 32 with permission from Biosens. Bioelectron.). | ||
In addition, nanozymes can be used to diagnose neurodegenerative diseases based on their peroxidase activity. Chang et al. designed a highly sensitive and selective fluorescent assay for acetylcholine (ACh) based on enzyme-mimicking Au/Ag nanoparticles (NPs).33 The colorimetric detection of acetylcholine provided a new idea for the diagnosis of neurodegenerative diseases.
In conclusion, nanozymes can be used to diagnose many diseases, such as cancer, metabolic diseases and infectious diseases. Peroxidase activity has mainly been used in disease diagnosis, followed by oxidase activity, catalase activity and SOD activity (Table 1). Nanozymes have the characteristics of stable activity, simple preparation, easy modification and amplification sensitivity, which can be well applied to the diagnosis of diseases, especially the early diagnosis of diseases and their early treatment.
| Nanomaterial | Activity | Application | Reference |
|---|---|---|---|
| PtNPs/GO | POD | Detect cancer cells | 22 |
| g-C3N4 NSs | POD | Detect breast cancer cells | 23 |
| M-HFn | POD | Detect tumor tissues | 24 |
| GQDzyme/ABTS | POD | In vivo photoacoustic imaging | 26 |
| AuNCs | POD | Nanosensor | 27 |
| SPIO@GCS/acryl/biotin-CAT/SOD-gel | CAT, SOD | Magnetic resonance imaging | 28 |
| NGQDs | POD | Cancer detection | 29 |
| GO (GO-COOH) | POD | Detect glucose in the blood | 10 |
| MNPs | POD | Detect uric acid in human serum | 30 |
| BSA-Ag NCs | OXD | Detect HIV virus | 31 |
| Fe3O4 magnetic nanoparticle (MNP) | POD | Detect Ebola virus | 32 |
| Au/Ag NPs | POD | Detect neurodegenerative diseases | 33 |
2 Nanozyme-based disease therapy
Nanozymes can simulate the catalytic processes of natural enzymes and regulate the redox level of cells, especially on reactive oxygen species (ROS). The multi-enzymatic activity of nanozymes can be used in the treatment of diseases, such as cancer, and diseases related to free radicals and bacterial infections.2.1 Nanozymes in cancer therapy
Cancer is a disease with a high death rate and is difficult to cure. Current treatments for cancer are as follows: surgical treatment, radiotherapy, chemotherapy and biological therapy. Due to their side effects and poor prognosis, it is difficult to meet clinical needs. Compared with natural enzymes, nanozymes have attracted extensive interest due to their high stability, low cost and easy preparation, especially in the field of cancer therapy.14,34 Nanozymes can target tumor cells by EPR effects and targeting peptides. Nanozymes kill cancer cells in two ways. The first is direct killing, by increasing ROS in vivo through the oxidase and peroxidase35 activities of nanozymes. The second is indirect killing, which is related to the catalase36 or SOD37 activity of nanozymes by relieving the hypoxia of a tumor microenvironment, aided by light, sound or chemotherapy.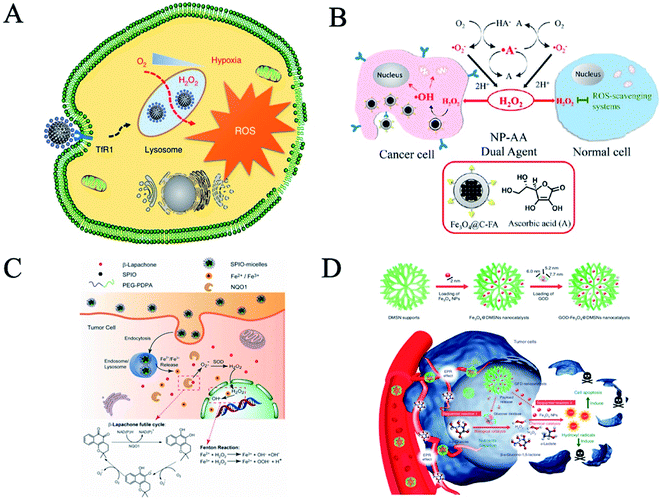 | ||
| Fig. 4 Nanozyme-based tumor catalytic therapy. (A) Schematic for N-PCNS-induced tumor cell destruction via ferritin-mediated specific delivery (reprinted from ref. 38 with permission from Nat. Commun.). (B) Schematic illustration of oxidative-stress-induced cytotoxicity to different cells using a combination of Fe3O4@C-FA NPs and AA (reprinted from ref. 39 with permission from ACS Appl. Mater. Interfaces). (C) Intracellular distribution of pH-sensitive SPION-micelles, iron ion release, subsequent Fenton reactions with NQO1-dependent H2O2 generated from β-lapachone (β-lap) futile redox cycle to amplify ROS stress levels for improved antitumor efficacy (reprinted from ref. 40 with permission from Theranostics). (D) Fabrication and catalytic-therapeutic schematics of sequential GFD NCs (reprinted from ref. 41 with permission from Nat. Commun.). | ||
Nanozymes can synergistically enhance ROS and improve the efficacy of tumor killing. Ascorbic acid (AA) can interfere with the normal redox state of cells, inhibit the growth of cancer cells and cause toxic effects by producing large amounts of reactive oxygen species (ROS). However, tolerable doses of AA are less effective in vivo when applied clinically. An et al. developed a peroxidase-like composite nanoparticle for an AA-mediated therapeutic strategy.39 They synthesized Fe3O4@C nanoparticles (NPs) and then modified them with folic acid (FA) on the surface (Fig. 4B). The carbon shell of Fe3O4@C NPs contains some graphitized carbon, which helps H2O2 catalyze the process of electron transfer in decomposition, thus leading to the production of highly reactive hydroxyl radicals and enhancing ROS. Fe3O4@C-FA NPs show magnetic responsiveness and receptor-binding specificity, and the peroxidase-like catalytic activity significantly promotes AA-induced oxidative stress in cancer cells and optimizes the anti-tumor efficacy of ROS-mediated exogenous AA.
Superparamagnetic iron oxide nanoparticles (SPION) are an important and versatile nano-platform due to their peroxidase activity. Huang et al. used the peroxidase activity of SPION to generate ROS and combined them with β-lapachone (β-lap), a novel anti-cancer drug that generates ROS, to produce a synergistic effect in treating tumors, increasing the therapeutic index of β-lap 10-fold by enhancing oxidative stress in cancer cells (Fig. 4C).40
A tumor microenvironment provides unique physical and chemical conditions for selective tumor therapy. Here, Shi et al. introduced the concept of sequential catalytic nano drugs. GOD-Fe3O4@DMSN (GFD NCs) were prepared by loading natural glucose oxidase (GOD, an enzyme catalyst) and ultra-small Fe3O4 nanoparticles (an inorganic nanozyme catalyst) into degradable large-pore dendritic silica nanoparticles (DMSN) (Fig. 4D).41 GOD-Fe3O4@DMSN (GFD NCs) were transported to the tumor site, then transformed into hydroxyl radicals with high efficiency and toxicity in situ, which then led to the apoptosis of the tumor cells, under the stimulation of excessive glucose nutrition in the tumor microenvironment and the slightly acidic metabolic environment.
The contents of metabolites such as acid and hydrogen peroxide in a tumor microenvironment are higher than those of normal tissues. In addition, tumor hypoxia is also characteristic of solid tumors. It not only increases the resistance of tumor cells to chemotherapy, but is also the main cause of tumor distant metastasis. Li et al. constructed a novel nanozyme with tumor microenvironment responsiveness (PtFe@Fe3O4).34 As a hydrogen peroxide (H2O2)-responsive nanozyme, PtFe@Fe3O4 can decompose H2O2 to produce oxygen and hydroxyl radicals at the same time, overcome the hypoxic environment of the tumor and effectively kill off tumor cells. In addition, in the process of PtFe@Fe3O4 nanozyme catalysis, Fe3O4 acts as an electron pump to keep Pt in the electron-rich state, thus enhancing catalytic activity. Under the stimulation of near-infrared light, the catalytic activity of PtFe is further improved due to the presence of surface plasmon resonance. In a treatment experiment in vivo, the enzyme activity was enhanced by means of fiber intervention, and the photothermal effect of the material itself was combined to enhance the efficient treatment of deep pancreatic cancer.
Zhou et al. developed a nanosystem by engineering nanozymes combining tumor starvation and low-temperature photothermal therapy.42 Glucose oxidase (GOx) was loaded within porous hollow Prussian Blue nanoparticles (PHPBNs) which were then coated with hyaluronic acid (HA) via redox-cleavable linkage, thereby allowing the nanocarrier to bind specifically to CD44-overexpressing tumor cells. In the hypoxic tumor microenvironment, the photothermal properties of PHPBNs (Fig. 5A) can directly kill the tumor as well as catalyze the decomposition of H2O2 to generate O2 to avoid hypoxia-induced photothermal resistance. GOx-loaded PHPBNs can induce tumor starvation for further collaborative treatment. Furthermore, this system may consequently suppress the expression of heat shock proteins (HSPs) after photothermal treatment to reduce their resistance to the PHPBN-mediated low-temperature photothermal therapy.
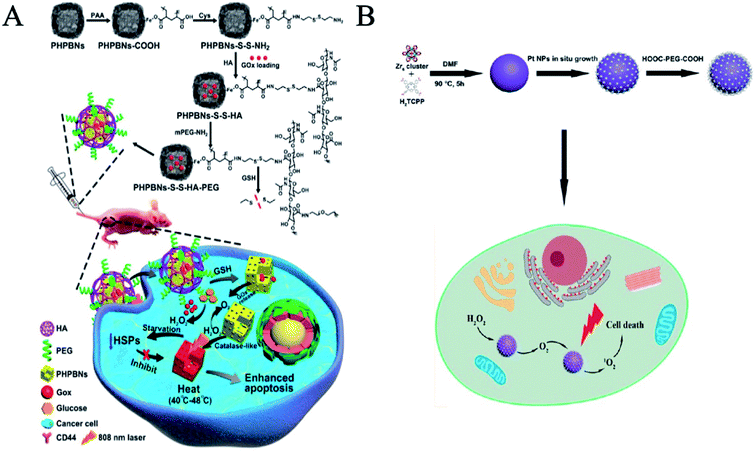 | ||
| Fig. 5 Nanozymes for relieving hypoxia in tumor therapy. (A) Synthesis scheme of PHPBNs-S-S-HA-PEG@GOx and illustration of GOx-induced starvation for enhanced low-temperature photothermal therapy in a hypoxic tumor microenvironment (reprinted from ref. 42 with permission from ACS Nano). (B) PCN-Pt NPs enhanced PDT (reprinted from ref. 44 with permission from ACS Nano). | ||
Photodynamic therapy (PDT) is a new method of treating tumors with photosensitive drugs and laser activation. A laser with specific wavelengths irradiating tumor sites activates photosensitive drugs that selectively accumulate in tumor tissues, triggering photochemical reactions that destroy the tumors. However, hypoxia is often present in tumor tissues, which limits the effectiveness of photodynamic therapy. Nanozymes can increase the oxygen content in tumor sites through catalase activity and achieve the purpose of synergistic enhancement therapy. Zhu et al. used manganese dioxide (MnO2) nanoparticles to catalyze endogenous hydrogen peroxide (H2O2) to generate O2 with high reactivity in the tumor microenvironment, regulate the tumor hypoxic microenvironment, and enhance tumor-specific photodynamic therapy (PDT).43 Zhang et al. reported a simple and universal strategy to enhance PDT by decorating platinum nanozymes onto photosensitizer-integrated MOF (Fig. 5B).44 Platinum nanoparticles uniformly fixed on MOF have high stability and catalase-like activity, which can promote the formation of O2 and the release of activated O2 in hypoxic tumor sites through H2O2, thus causing more serious damage to cancer cells. Moreover, Liu et al. found that amino terminal PAMAM dendritic-coated gold nanoclusters (AuNCs-NH2) can produce O2 for PDT via their intrinsic catalase-like activity for effective anticancer treatments.36
Tumor radiotherapy (RT) is a local tumor treatment method using radiation. After entering the cell, the nanozyme has a different localization and shows different activity in the pH microenvironment. Wason et al. reported that the CAT activity of cerium oxide nanoparticles (CONPs) was suppressed in acidic conditions, whereas the SOD activity was not affected, so that the CeO2 nanozymes can be used as a radiotherapy (RT) sensitizer.37
2.2 Nanozymes as antioxidants
Reactive oxygen species (ROS) are intermediate products that emerge in the process of oxygen metabolism, mainly including superoxide anion (O2˙−), hydroxyl radical (·OH) and hydrogen peroxide (H2O2).13 A high concentration of active oxygen free radicals can carboxylate or denature protein.45 An abnormal rise in ROS level will destroy the homeostasis of redox in vivo and cause oxidative stress, which destroys the structure and function of cellular macromolecules, such as proteins, lipids, and nucleic acids. Myocardial infarction,46 inflammation,47 aging48 and cancer are all related to the accumulation of ROS in vivo and the decrease in the level of antioxidant enzymes. The peroxidase activity and superoxide dismutase activity of nanozymes are mainly used to regulate the level of intracellular ROS, which play an important role in protecting cells.Parkinson's disease (PD) is a common degenerative disease of the nervous system, which is more common in the elderly. Oxidative stress may be involved in the process of degeneration and death of PD dopaminergic neurons. Nanozymes can alleviate the damage caused by oxidative stress. Fullerene, known as a free radical sponge, is the first carbon-based nanozyme that has been found to have SOD activity and has been used in humans. Dugan et al. found that C3-modified C60 improved the water solubility of fullerenes. After C3 treatment, Parkinson's movement scores in monkeys improved, and striatal dopamine levels increased.49 Namrata Singh et al. first applied Mn3O4 nanozymes in vivo, which showed superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activity.50 Single Mn3O4 nanoflowers have a high Mn2+/Mn3+ ratio, and large specific surface area and pore diameter. The anti-oxidation stability and enzyme activity of single Mn3O4 nanoflowers can effectively remove intracellular ROS and alleviate Parkinson's disease. Kwon et al. used CeO2, boosting both SOD and CAT enzyme activities, to inhibit the activation of microglia cells and lipid peroxidation by removing ROS inside and outside the cells, and to protect tyrosine hydroxylase for the treatment of Parkinson's disease.51
Inflammation is one of the body's defense responses to stimuli, and nanozymes are currently being used to fight inflammation. Arya et al. first applied CeO2 as an anti-inflammatory.47 They found that CeO2 was deposited in the lungs after being injected intraperitoneally and reduced reactive oxygen species and lipid oxidation, thereby protecting the lungs from oxidative stress caused by hypoxia and tissue damage caused by inflammation. Wei et al. proved that Mn3O4 nanoparticles (NPs) had superoxide dismutase activity, catalase activity and free radical scavenging functions.52 They are more stable than the corresponding natural enzymes, and superior to CeO2 nanozymes in ROS scavenging. Mn3O4 NPs have been demonstrated to not only remove ROS in vitro, but they also effectively protect live mice from ROS-induced otitis in vivo (Fig. 6A). In addition, Wei et al. found that copper tannic acid coordination (CuTA) nanozyme reduces oxidative stress pulmonary inflammation which can result in acute lung injury.53 CuTA is a highly active and heat-resistant reactive oxygen scavenger with superoxide dismutase-like activity, catalase-like activity, and hydroxyl radical elimination capacity. These characteristics can be applied to cigarette filters to reduce the toxic effects of cigarette smoke.
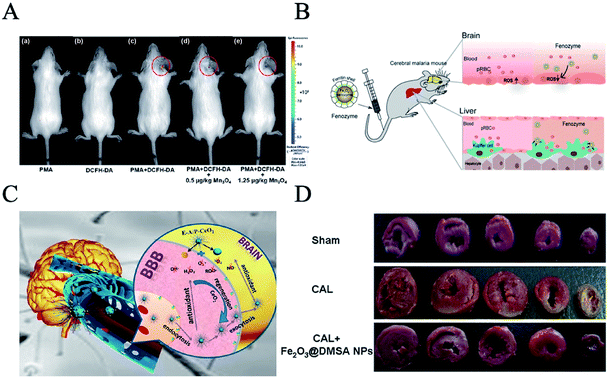 | ||
| Fig. 6 Nanozymes as antioxidants. (A) TEM image of Mn3O4 NPs and in vivo fluorescence imaging of mice with PMA-induced ear inflammation after treatment with (a) PMA, (b) DCFH-DA, (c) PMA and DCFH-DA, (d) PMA and DCFH-DA with 0.5 mg kg−1 Mn3O4 NPs, and (e) PMA and DCFH-DA with 1.25 mg kg−1 Mn3O4 NPs (reprinted from ref. 52 with permission from Chem. Sci.). (B) CeO2-loaded edaravone (a brain free radical scavenger), coupled with targeted peptides, can relieve stroke through BBB (reprinted from ref. 54 with permission from ACS Nano). (C) Fenozyme protects the integrity of the blood–brain barrier against experimental cerebral malaria (reprinted from ref. 55 with permission from Nano Lett.). (D) Fe2O3@DMSA NPs protected coronary artery ligature (CAL)-induced injury in rats (reprinted from ref. 46 with permission from Sci. Rep.). | ||
Cerium dioxide nanozyme is very effective in nerve protection. Shi et al. developed an effective stroke treatment agent based on monodispersed ceria nanoparticles, which are loaded with edaravone (a brain free radical scavenger) and modified with Angiopep-2 and poly (ethylene glycol) on their surface (E-A/P-CeO2) (Fig. 6B).54 CeO2-loaded edaravone, coupled with targeted peptides, can relieve stroke through the blood–brain barrier (BBB). In addition, Fenozyme is recombinant human ferritin (HFn) protein shell, the inner Fe3O4 nanozyme core exhibiting catalase-like activity (Fig. 6C).55 Fenozyme can remove reactive oxygen species by targeting brain endothelial cells and regulating nanozymes to treat malignant cerebral malaria. Animal experiments showed that Fenozyme can ameliorate the damage to the BBB induced by the parasite, reduce brain inflammation, and improve the survival rate of infected mice.
Gu et al. reported that the down-regulation of intracellular ROS by Fe2O3 could alleviate the damage to the heart caused by ischemia and protect cardiomyocytes (Fig. 6D).46 At the same time, Fe2O3 NPs showed no significant toxicity to normal cardiomyocytes.
2.3 Antibacterial nanozymes
Bacterial infection continues to be a growing global health problem with the most widely accepted treatment paradigms restricted to antibiotics,56 afflicting millions of people annually.57–59 At present, the emergence of new infectious diseases and bacterial resistance caused by antibiotics has become a worldwide problem and a major threat to global public health.58,60 To slow down the evolution of drug resistance and reduce the side effects caused by nonspecific bactericidal action, scientists have developed strain-selective bactericidal strategies.61 The antibacterial activity of nanozymes is beneficial to the development of new antibacterial agents. The antibacterial mechanism of nanozymes depends mainly on the activities of peroxidase and oxidase to catalyze the decomposition of H2O2 into ·OH to regulate ROS.Graphene and its derivatives have high antibacterial activity. Graphene quantum dots (GQDs) are a carbon nanomaterial with peroxidase-like activity.56 GQDs catalyze the decomposition of H2O2 to produce ·OH. Due to the high antibacterial activity of ·OH, the conversion of H2O2 into ·OH improves the antibacterial performance of H2O2, which makes it possible to avoid the toxicity of H2O2 in wound disinfection at a high level (Fig. 7A). GQDs can enhance the antibacterial activity of H2O2, and the designed system has a wide range of antibacterial activities against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria. GQDs can be applied to antibacterial band-aids. Then, they found that mesoporous silica-supported gold nanoparticles were sterilized by their intrinsic oxidase and peroxidase activities.62 At the same time, Pt/Ag nanoparticles, synthesized by Wang et al., can also play an efficient role in sterilization at a low concentration of H2O2.63 In addition, Pt hollow nanodendrites exert striking peroxidase-like activity due to the maximized utilization efficiency of the Pt atoms and the presence of high-index facets on their surface.64 Pt hollow nanodendrites exhibited improved antibacterial ability, which is conducive to wound healing.
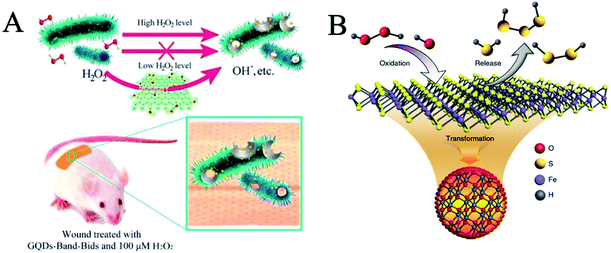 | ||
| Fig. 7 Antibacterial nanozymes. (A) Schematic illustration of the GQD-assisted antibacterial system (reprinted from ref. 56 with permission from Acc. Chem. Res.). (B) Scheme of polysulfane released from nFeS (reprinted from ref. 65 with permission from Nat. Commun.). | ||
Gao et al. extracted a variety of natural organic sulfides from garlic and converted them into nano iron sulfides with high antibacterial activity.65 Compared with natural organic sulfides, nanometer iron sulfide showed broad spectrum, high efficiency and bactericidal activity. Nano iron sulfides kill bacteria by releasing hydrogen polysulfide (Fig. 7B). In addition, nano iron sulfide is a nanozyme, possessing peroxidase and catalase activities, which can catalyze the decomposition of hydrogen peroxide. These enzyme-like catalytic activities can further accelerate the release of hydrogen polysulfide, thus enhancing the bactericidal effect. Nanometer iron sulfide can not only kill a variety of Gram-negative bacteria, but also significantly inhibit Gram-positive bacteria, such as oral Streptococcus mutants, Staphylococcus aureus and their drug-resistant strains (MRSA and MDR). In addition, it can also effectively destroy the caries on human teeth membrane as well as accelerate the healing of infected wounds.
Bacteria in natural, clinical, and industrial settings tend to attach to surfaces and organize themselves into multicellular communities known as biofilms.66 Dental biofilm (plaque) is very difficult to remove or treat, but antibacterial drugs can kill the microbes inside the plaque biofilm. Gao et al. found that catalytic nanoparticles (CAT-NPs) can be used to control plaque biofilm.67 CAT-NP activity is similar to the activity of peroxidase, which can trigger degradation of the extracellular matrix, and cause bacterial death within acidic niches of caries-causing biofilm. Then, CAT-NPs containing biocompatible Fe3O4 were designed to catalyze H2O2 to generate free radicals in situ so as to degrade the biofilm matrix and quickly kill the embedded bacteria. This method kills bacteria and prevents tooth decay.
Until now, nanozymes have been widely used in the field of disease treatment, including the therapies for cancer, Parkinson's and neurodegenerative diseases, to relieve strokes, to reduce inflammation, and as anti-bacterial agents. In terms of disease treatment, the peroxidase activity of nanozymes is the main use, followed by SOD activity, catalase activity and oxidase activity (Table 2).
| Nanomaterial | Activity | Application | Reference |
|---|---|---|---|
| N-PCNSs | POD, OXD, CAT, SOD | Tumor catalytic therapy | 38 |
| Fe3O4@C-FA NPs | POD | Enhance ROS and improve the efficacy of tumor killing | 39 |
| SPION | POD | Generate ROS to improve anticancer drug efficacy | 40 |
| GOD-Fe3O4@DMSN | Fenton-like reaction | Tumor therapy | 41 |
| PtFe@Fe3O4 | POD, CAT | Pancreatic cancer therapy | 34 |
| PHPBNs-S-S-HA-PEG@GOx | CAT | Tumor starvation and low-temperature photothermal therapy | 42 |
| MnO2 NPs | POD | Enhance photodynamic therapy | 43 |
| Pt NPs | CAT | Enhance photodynamic therapy | 44 |
| AuNCs-NH2 | CAT | Enhance photodynamic therapy | 36 |
| CeO2 | CAT | Radiotherapy (RT) sensitizer | 37 |
| C3-modified C60 | SOD | Parkinson's disease | 49 |
| Mn3O4 | SOD, CAT, GPx | Parkinson's disease | 50 |
| CeO2 | SOD, CAT | Parkinson's disease | 51 |
| CeO2 | SOD, CAT | Anti-inflammatory | 47 |
| Mn3O4 NPs | SOD, CAT | Anti-inflammatory | 52 |
| CuTA | SOD, CAT | Scavenging ROS from cigarette smoke | 53 |
| E-A/P-CeO2 | SOD, CAT | Nerve protection | 54 |
| Fe2O3@DMSA NPs | SOD | Treat cardiovascular diseases | 46 |
| GQDs | POD | Antibacterial band-aids | 56 |
| Pt/Ag nanoparticles | POD | Anti-bacterial | 63 |
| Pt hollow | POD | Anti-bacterial, wound healing | 64 |
| nFeS | POD, CAT | Anti-bacterial, prevent tooth decayed, wound healing | 65 |
| CAT-NPs | POD | Anti-bacterial, control plaque biofilm | 67 |
3 Conclusions and prospects
Inspired by nature,2 nanozymes have many advantages and are widely used in the diagnosis and treatment of diseases. In this review, we have highlighted the applications of nanozymes in biomedicine, including diagnosis and treatment. Nanozymes can diagnose cancer, metabolic diseases, neurodegenerative diseases etc., depending on their peroxidase activity, catalase activity and oxidase activity. In terms of disease treatment, nanozymes can be used for cancer treatment, antioxidation and antibacterial treatment, based on their peroxidase activity, catalase activity, oxidase activity and SOD activity.Nanozymes are a new generation of artificial enzyme mimics. Nanozymes have strong stability, excellent designability and diverse functions, and are expected to replace natural enzymes in the future and be applied more accurately in the medical field. However, some issues need to be addressed. Firstly, the enzyme activities of nanozymes are mainly redox enzymes, which are less widely used than natural enzymes. Secondly, the catalytic efficiency of nanozymes is not up to the level of natural enzymes. Thirdly, the applications of nanozymes in disease diagnosis and treatment are still in the initial stages. Whether the toxicity of the material itself will limit the applications of nanozymes in biomedicine is still in question. These problems are both challenges and opportunities for nanozymes.
Conflicts of interest
The author(s) declare that they have no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31530026, 31900981, 31871005), Chinese Academy of Sciences under Grant No. YJKYYQ20180048, the Strategic Priority Research Program (XDPB29040101), the Key Research Program of Frontier Sciences, CAS (Grant No. QYZDY-SSW-SMC013), National Key Research and Development Program of China (No. 2017YFA0205501) and Youth Innovation Promotion Association CAS (2019093).References
- X. Wang, Y. Hu and H. Wei, Inorg. Chem. Front., 2016, 3, 41–60 RSC.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- H. Dong, Y. Fan, W. Zhang, N. Gu and Y. Zhang, Bioconjugate Chem., 2019, 30, 1273–1296 CrossRef CAS PubMed.
- D. P. Cormode, L. Gao and H. Koo, Trends Biotechnol., 2018, 36, 15–29 CrossRef CAS PubMed.
- X. Meng, K. Fan and X. Yan, Sci. China: Life Sci., 2019, 62, 1543–1546 CrossRef PubMed.
- B. Jiang, L. Fang, K. Wu, X. Yan and K. Fan, Theranostics, 2020, 10, 687–706 CrossRef.
- L. Gao, K. Fan and X. Yan, Theranostics, 2017, 7, 3207–3227 CrossRef CAS PubMed.
- C. Xu and X. Qu, NPG Asia Mater., 2014, 6, e90 CrossRef CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- H. Sun, Y. Zhou, J. Ren and X. Qu, Angew. Chem., Int. Ed. Engl., 2018, 57, 9224–9237 CrossRef CAS PubMed.
- Y. Lin, J. Ren and X. Qu, Adv. Mater., 2014, 26, 4200–4217 CrossRef CAS PubMed.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- Y. Lin, J. Ren and X. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS PubMed.
- B. Abel, B. Odukoya, M. Mohammed and K. Aslan, Nano Biomed. Eng., 2015, 7, 92–101 CAS.
- L. Fan, Y. Tian, R. Yin, D. Lou, X. Zhang, M. Wang, M. Ma, S. Luo, S. Li, N. Gu and Y. Zhang, Nanoscale, 2016, 8, 8553–8558 RSC.
- Y. Zheng, W. Liu, Z. Qin, Y. Chen, H. Jiang and X. Wang, Bioconjugate Chem., 2018, 29, 3094–3103 CrossRef CAS PubMed.
- N. Yu, T. Cai, Y. Sun, C. Jiang, H. Xiong, Y. Li and H. Peng, Int. J. Pharm., 2018, 552, 277–287 CrossRef CAS PubMed.
- L.-Z. Gao and X.-Y. Yan, Acta Agron. Sin., 2013, 40, 892–902 CrossRef CAS.
- L. N. Zhang, H. H. Deng, F. L. Lin, X. W. Xu, S. H. Weng, A. L. Liu, X. H. Lin, X. H. Xia and W. Chen, Anal. Chem., 2014, 86, 2711–2718 CrossRef CAS PubMed.
- Y. M. Wang, J. W. Liu, G. B. Adkins, W. Shen, M. P. Trinh, L. Y. Duan, J. H. Jiang and W. Zhong, Anal. Chem., 2017, 89, 12327–12333 CrossRef CAS PubMed.
- K. Fan, C. Cao, Y. Pan, D. Lu, D. Yang, J. Feng, L. Song, M. Liang and X. Yan, Nat. Nanotechnol., 2012, 7, 459–464 CrossRef CAS PubMed.
- J. He, K. Fan and X. Yan, J. Controlled Release, 2019, 311–312, 288–300 CrossRef CAS PubMed.
- H. Ding, Y. Cai, L. Gao, M. Liang, B. Miao, H. Wu, Y. Liu, N. Xie, A. Tang, K. Fan, X. Yan and G. Nie, Nano Lett., 2019, 19, 203–209 CrossRef CAS PubMed.
- C. N. Loynachan, A. P. Soleimany, J. S. Dudani, Y. Lin, A. Najer, A. Bekdemir, Q. Chen, S. N. Bhatia and M. M. Stevens, Nat. Nanotechnol., 2019, 14, 883–890 CrossRef CAS PubMed.
- X. Wang, D. C. Niu, P. Li, Q. Wu, X. W. Bo, B. J. Liu, S. Bao, T. Su, H. X. Xu and Q. G. Wang, ACS Nano, 2015, 9, 5646–5656 CrossRef CAS PubMed.
- J. Xi, C. Xie, Y. Zhang, L. Wang, J. Xiao, X. Duan, J. Ren, F. Xiao and S. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 22563–22573 CrossRef CAS PubMed.
- A. Fang, Q. Wu, Q. Lu, H. Chen, H. Li, M. Liu, Y. Zhang and S. Yao, Biosens. Bioelectron., 2016, 86, 664–670 CrossRef CAS PubMed.
- G. L. Wang, L. Y. Jin, X. M. Wu, Y. M. Dong and Z. J. Li, Anal. Chim. Acta, 2015, 871, 1–8 CrossRef CAS PubMed.
- D. Duan, K. Fan, D. Zhang, S. Tan, M. Liang, Y. Liu, J. Zhang, P. Zhang, W. Liu, X. Qiu, G. P. Kobinger, G. Fu Gao and X. Yan, Biosens. Bioelectron., 2015, 74, 134–141 CrossRef CAS PubMed.
- C. I. Wang, W. T. Chen and H. T. Chang, Anal. Chem., 2012, 84, 9706–9712 CrossRef CAS PubMed.
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu, Q. Wu, Y. Sun, Q. Zhang, H. Yang, F. Zhang, L. Gu, T. Zhang and H. Liu, Angew. Chem., Int. Ed. Engl., 2019, 58, 12624–12631 CrossRef CAS PubMed.
- D. Zhang, Y.-X. Zhao, Y.-J. Gao, F.-P. Gao, Y.-S. Fan, X.-J. Li, Z.-Y. Duan and H. Wang, J. Mater. Chem. B, 2013, 1, 1–8 Search PubMed.
- C. P. Liu, T. H. Wu, C. Y. Liu, K. C. Chen, Y. X. Chen, G. S. Chen and S. Y. Lin, Small, 2017, 13, 1–9 Search PubMed.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu, Y. Tang, X. Xu, M. Liang, B. Jiang, X. Yan and L. Gao, Nat. Commun., 2018, 9, 1440 CrossRef PubMed.
- Q. An, C. Sun, D. Li, K. Xu, J. Guo and C. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 13248–13257 CrossRef CAS PubMed.
- G. Huang, H. Chen, Y. Dong, X. Luo, H. Yu, Z. Moore, E. A. Bey, D. A. Boothman and J. Gao, Theranostics, 2013, 3, 116–126 CrossRef CAS PubMed.
- M. Huo, L. Wang, Y. Chen and J. Shi, Nat. Commun., 2017, 8, 357 CrossRef PubMed.
- J. Zhou, M. Li, Y. Hou, Z. Luo, Q. Chen, H. Cao, R. Huo, C. Xue, L. Sutrisno, L. Hao, Y. Cao, H. Ran, L. Lu, K. Li and K. Cai, ACS Nano, 2018, 12, 2858–2872 CrossRef CAS PubMed.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef CAS.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 651–661 CrossRef CAS PubMed.
- A. A. Vernekar, D. Sinha, S. Srivastava, P. U. Paramasivam, P. D'Silva and G. Mugesh, Nat. Commun., 2014, 5, 5301 CrossRef CAS PubMed.
- F. Xiong, H. Wang, Y. Feng, Y. Li, X. Hua, X. Pang, S. Zhang, L. Song, Y. Zhang and N. Gu, Sci. Rep., 2015, 5, 8579 CrossRef CAS PubMed.
- A. Arya, N. K. Sethy, S. K. Singh, M. Das and K. Bhargava, Int. J. Nanomed., 2013, 8, 4507–4520 Search PubMed.
- Y. Zhang, Z. Wang, X. Li, L. Wang, M. Yin, L. Wang, N. Chen, C. Fan and H. Song, Adv. Mater., 2016, 28, 1387–1393 CrossRef CAS PubMed.
- L. L. Dugan, L. Tian, K. L. Quick, J. I. Hardt, M. Karimi, C. Brown, S. Loftin, H. Flores, S. M. Moerlein, J. Polich, S. D. Tabbal, J. W. Mink and J. S. Perlmutter, Ann. Neurol., 2014, 76, 393–402 CrossRef CAS PubMed.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Angew. Chem., Int. Ed. Engl., 2017, 56, 14267–14271 CrossRef CAS PubMed.
- H. J. Kwon, D. Kim, K. Seo, Y. G. Kim, S. I. Han, T. Kang, M. Soh and T. Hyeon, Angew. Chem., Int. Ed. Engl., 2018, 57, 9408–9412 CrossRef CAS PubMed.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Li and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- S. Lin, Y. Cheng, H. Zhang, X. Wang, Y. Zhang, Y. Zhang, L. Miao, X. Zhao and H. Wei, Small, 2019, e1902123, DOI:10.1002/smll.201902123.
- Q. Bao, P. Hu, Y. Xu, T. Cheng, C. Wei, L. Pan and J. Shi, ACS Nano, 2018, 12, 6794–6805 CrossRef CAS PubMed.
- S. Zhao, H. Duan, Y. Yang, X. Yan and K. Fan, Nano Lett., 2019, 19, 8887–8895 CrossRef CAS PubMed.
- Z. Chen, Z. Wang, J. Ren and X. Qu, Acc. Chem. Res., 2018, 51, 789–799 CrossRef CAS PubMed.
- S. Chen, Y. Quan, Y.-L. Yu and J.-H. Wang, ACS Biomater. Sci. Eng., 2017, 3, 313–321 CrossRef CAS.
- G. Fang, W. Li, X. Shen, J. M. Perez-Aguilar, Y. Chong, X. Gao, Z. Chai, C. Chen, C. Ge and R. Zhou, Nat. Commun., 2018, 9, 129 CrossRef PubMed.
- Z. Wang, K. Dong, Z. Liu, Y. Zhang, Z. Chen, H. Sun, J. Ren and X. Qu, Biomaterials, 2017, 113, 145–157 CrossRef CAS PubMed.
- W. Yin, J. Yu, F. Lv, L. Yan, L. R. Zheng, Z. Gu and Y. Zhao, ACS Nano, 2016, 10, 11000–11011 CrossRef CAS PubMed.
- J. Niu, Y. Sun, F. Wang, C. Zhao, J. Ren and X. Qu, Chem. Mater., 2018, 30, 7027–7033 CrossRef CAS.
- Y. Tao, E. Ju, J. Ren and X. Qu, Adv. Mater., 2015, 27, 1097–1104 CrossRef CAS PubMed.
- S. Cai, X. Jia, Q. Han, X. Yan, R. Yang and C. Wang, Nano Res., 2017, 10, 2056–2069 CrossRef CAS.
- C. Ge, R. Wu, Y. Chong, G. Fang, X. Jiang, Y. Pan, C. Chen and J.-J. Yin, Adv. Funct. Mater., 2018, 28, 1–11 Search PubMed.
- Z. Xu, Z. Qiu, Q. Liu, Y. Huang, D. Li, X. Shen, K. Fan, J. Xi, Y. Gu, Y. Tang, J. Jiang, J. Xu, J. He, X. Gao, Y. Liu, H. Koo, X. Yan and L. Gao, Nat. Commun., 2018, 9, 3713 CrossRef CAS PubMed.
- Z. Chen, H. Ji, C. Liu, W. Bing, Z. Wang and X. Qu, Angew. Chem., Int. Ed. Engl., 2016, 55, 10732–10736 CrossRef CAS PubMed.
- L. Gao, Y. Liu, D. Kim, Y. Li, G. Hwang, P. C. Naha, D. P. Cormode and H. Koo, Biomaterials, 2016, 101, 272–284 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
