Open Access Article
Open Access ArticleStudy of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of pH
Petra Suchánková ,
Ekaterina Kukleva
,
Ekaterina Kukleva ,
Karel Štamberg,
Pavel Nykl,
Martin Vlk
,
Karel Štamberg,
Pavel Nykl,
Martin Vlk and
Ján Kozempel
and
Ján Kozempel *
*
Czech Technical University in Prague, Faculty of Nuclear Sciences and Physical Engineering, Department of Nuclear Chemistry, Břehová 7, 11519 Prague 1, Czech Republic. E-mail: jan.kozempel@fjfi.cvut.cz
First published on 22nd January 2020
Abstract
The mechanism of 223Ra uptake on hydroxyapatite and titanium dioxide nanoparticles was studied as a function of pH. Both materials are widely used in food industry and medicine. They offer properties suitable for labelling with medicinal radionuclides, particularly for targeted radionuclide therapy. The selected isotope, 223Ra, is an alpha emitter widely used in targeted alpha particle therapy due to high-dose delivery in very small tissue volume, nevertheless the results are applicable for any radium isotope including 226Ra. The study was performed in the pH range 4.5 to 12 for hydroxyapatite nanoparticles and 2 to 12 for titanium dioxide nanoparticles in Britton–Robinson buffer solution. Both nanomaterials at pH 6 and higher showed that over 95% of the radium has been sorbed. According to the applied chemical equilibrium model, the most important species playing a role in sorption on the edge-sites were RaCO3, RaPO4−, RaHPO4 and Ra(Ac−)2, and Ra2+ and RaH2PO4+ on layer-sites. All experiments were conducted under free air conditions and no negative impact of CO2 was found. The surface complexation model was found suitable for describing radium uptake by the studied hydroxyapatite and titanium dioxide nanomaterials.
Introduction
Hydroxyapatite (HAp) and titanium dioxide are materials intensely studied and widely used for their favorable properties in many fields. Due to this, the range of their applications is extended from foodstuffs, cosmetics and sunscreen creams through environmental decontamination to medicine, which is our area of interest. Both materials are biocompatible and have relatively low clinical toxicity (LD50, HAp (oral, rat) over 25 g kg−1; LD50, TiO2 (oral, rat) over 10 g kg−1), which is the underlying assumption for using them for patient applications.1 Some advantages of radionuclide sorption are the following properties: high specific surface area, radiation stability and size. Apart from the previously mentioned reasons, ease of synthesis on a nanoscale size is another argument for selection of these two materials.2–7Our main area of interest is specifically the possibility of the application of the chosen nanomaterials as a drug carrier system for diagnostic, therapeutic or theragnostic radionuclides. The targeting of the nanoparticles to the required tissue can be enabled by two mechanisms. The first one is passive targeting due to the Enhanced Permeability and Retention effect (EPR effect). This effect exploits the size of the drug carriers. Tumor tissue grows fast and stimulates angiogenesis. Conveniently, the novel blood vessels are abnormal and leaky, so the nanocarriers can get stuck in the tumor tissue.8–10 The second one is active targeting by functionalization of the drug carrier surface. The carriers can be modified by specific ligands or antigens and the drugs are delivered to the target tissue due to their bond to the required receptor.11 The basic principles of nanocarriers' preparation for the targeted alpha particle therapy (TAT), particularly bearing the alpha-emitting nuclides that decay in series, were described previously.12
Accordingly, hydroxyapatite (nHAp) and titanium dioxide nanoparticles (nTiO2) could be applied as useful vehicles for radionuclide delivery in case of radiolabelling with an appropriate radionuclide, such as 18F, 68Ga, 99mTc etc. for diagnostic purposes or 225Ac, 213Bi, 177Lu, 223Ra, 186Re, 90Y and others for therapeutic purposes.13–20 Nowadays, there are several publications dealing with radiolabelling of nHAp and nTiO2. For example, there are published studies, where the nHAp is labelled by alpha therapeutic radionuclide – 223Ra.12 Other radionuclides used for labelling were 134,137Cs or 90Sr, where the nHAp was applied for remediation of contaminated waters by anthropogenic radionuclides.21 Available published data for radiolabelling of nTiO2 are limited. There are some studies dedicated to labelling of TiO2 with 48V or 125I for in vivo toxicological studies22,23 and with 225Ac for targeted alpha radionuclide therapy.24
This article is focused on the study of 223Ra sorption on nHAp and nTiO2. The published data on radium sorption using chosen nanoparticles are quite limited and available papers are focused mainly on 226Ra behavior in uranium mill tailings and the articles studied only iron compounds as a goethite or ferrihydrite, or environmental minerals.25–27
The 223Ra is a radionuclide used for TAT as radium chloride (Xofigo®).12,28–30 Due to the cascade of emitted alpha particles, it could provide accurate high-dose irradiation of the target tissue (range of the alpha particles approx. 10 cells) without damage to the organism, if appropriate targeting strategy is applied. Nowadays, Xofigo® is used for the therapy of metastatic prostate cancer, but number of its applications decreases. Possible fields and ways of its applications could be significantly wider due to the capture of recoil nuclei by the nanoparticles and advantages of 223Ra. Furthermore, 223Ra can be obtained from a radionuclide generator 227Ac/227Th/223Ra,28–33 which can probably guarantee availability of the radionuclide in the hospitals. Main introduced disadvantage of 223Ra are recoil nuclei appearing during the radioactive decay. They have considerable energy, which means, that daughter nuclei are escaping from chemical bonds and are distributed into the organism due to their chemical and biological properties. In the case of 223Ra (α-emitter, T1/2 = 11.4 days), its daughter decay products with half-lives longer than one minute are: 211Pb (β−-emitter, T1/2 = 36.1 min), 211Bi (α-emitter, T1/2 = 2.1 min) and 207Tl (β−-emitter, T1/2 = 4.8 min).34
The aim of this work is focused on the study of 223Ra sorption as a function of pH. The mechanism of radium uptake by nHAp and nTiO2 in Britton–Robinson buffer solution (BRB) was modelled and absorbed species were studied. For describing the surface complexation systems, chemical equilibrium model (CEM) was used. Modelling programs and codes for study of sorption mechanism requires protonation constants, ion-exchange constants and total concentration of edge-sites and layer-sites, which were determined experimentally via titration method and corresponding models.35–37
Experimental
Materials
All chemicals were of analytical grade purchased from Merck Millipore (Germany) and were used without further purification: sodium hydroxide, tetrabutyl ortho-titanate (TBOT), 2-propanol (IPA), ammonium hydroxide, phosphoric acid, nitric acid, methanol, boric acid, acetic acid, calcium nitrate tetrahydrate and diammonium hydrogen phosphate. Demineralized water of 18 MΩ cm−1 was obtained from water purification system (Millipore, USA). The activities of the samples were measured with a well-type NaI(Tl) crystal detector (Capintec, USA). For mixing of samples, Stuart SSM3 rocker (Cole-Parmer Ltd, United Kingdom) was used and the separation was made on VWR Micro Star 12 centrifuge (VWR International, LLC, USA). Gamma spectra were recorded on Canberra Packard HPGe detector (USA) under GammaVision software.Britton–Robinson buffer solution
For Britton–Robinson buffer solution preparation in the pH ranging from 2 to 12, two stock solutions were mixed in appropriate ratio. The first one was 0.2 M sodium hydroxide and the second one was the mixture of 0.04 M phosphoric acid, 0.04 M boric acid and 0.04 M acetic acid.Preparation of 223Ra stock solution
The 223Ra was eluted from 227Ac/227Th/223Ra generator, which was prepared at our laboratory based on the study published by Guseva et al.33 The column of the generator was filled with 0.5 g of Dowex-1 × 8 and 227Ac in equilibrium with its decay products was loaded on it. The elution was provided by 0.7 M nitric acid in 80% methanol solution for the separation of 223Ra from 227Ac and 227Th. The eluted 223Ra(NO3)2 solutions were dried and reconstituted with deionized water. Possible breakthrough of parent radionuclides was checked by gamma spectrometry and was not observed in the eluate.Sorbent materials preparation
The sorbent preparation was described by Kukleva et al. in detail.35 In this article only brief procedure is mentioned.For hydroxyapatite nanoparticles preparation, 1.2 M Ca(NO3)2 (24 mL) was added into 0.5 L of demineralized water. It was necessary to set and maintain the pH of the mixture to 11 with ammonium hydroxide. Afterwards, the solution of calcium nitrate was stirred and 0.7 M (NH4)2HPO4 (24 mL) was added dropwise. The mixture was left overnight under stirring, washed three times with demineralized water (20 mL) and then dried under vacuum.
The titanium dioxide nanoparticles were prepared by the dropwise addition of the mixture of TBOT (1 mL) in IPA (4 mL) into demineralized water in ultrasonic generator and was stirred for 30 minutes. Prepared nTiO2 were washed three times with demineralized water (20 mL), once with IPA (10 mL) and then dried under vacuum.
Sorption experiments
Experiments were performed in pH range from 4.5 to 12 for nHAp and from 2 to 12 for nTiO2. All samples were in triplets and were prepared in a following way: 2 mg of nHAp or 5 mg of nTiO2 were dispersed in 1 mL of BRB. Then 50 μL of 223Ra(NO3)2 was dosed into each sample vial (pH of Ra solution was adjusted before adding to the sample by diluted ammonium hydroxide to the approx. sample pH in order not to exceed BRB's buffering capacity). Added radioactivity was ranged between 1.4 and 2.4 kBq. All samples were shaken for 24 hours, centrifuged and the supernatant was quantitatively removed. Then samples were dispersed in 1 mL of demineralized water in order to ensure same geometry of measurement. All samples and all supernatants were measured. All experiments were accomplished under free air conditions and at the laboratory temperature.In the same manner, sorption of pure 223Ra (without addition of nanomaterials) on the vial walls was studied in pH range from 2 to 12.
Modelling of the 223Ra uptake
First of all, calculations of 223Ra speciation diagrams were performed based on stability constants of Ra2+ mainly. In the case of unavailable Ra constants, Ba2+, Sr2+ and Ca2+ were used and were chosen due to their similar chemical properties. The stability constant values were taken from the Hatches database used in the geochemical code PhreeqC.38Then the model based on the surface complexation theory was constructed and incorporated into the simulation code. According to the surface complexation theory, it is supposed that there are two types of surface functional groups: edge-sites and layer-sites. Their concentrations were already calculated based on experimental data.35,36 For further modelling protonation constants of edge-sites, ion-exchange constant of layer-sites and the total concentration of both sites were used as input data.
The software product FAMULUS39 and our code Praspec6.fm (code package STAMB 2017) were used for the speciation calculations. The corresponding code, Pramg6ZM.fm (code package STAMB 2017), is based on the Newton–Raphson multidimensional nonlinear regression used for experimental data fitting. It has to be added, that the number of complexing reactions, which can be used in calculation, is selectable. The appropriateness of the fit is evaluated by the χ2-test, and the values of χ2 are used to the calculation of criterion WSOS/DF (weighted sum of squares divided by degrees of freedom).40 It holds, if 0.1 < WSOS/DF < 20, then there is a good agreement between the experimental and the calculated data.
Results and discussion
As mentioned above, published studies25–27 were focused only on 226Ra and the sorption was studied only on a few materials. For the medicinal purposes, the attention was paid to the sorption of 223Ra in BRB and it was experimentally studied on nHAp in the pH range of 4.5–12 and of 2–12 for nTiO2. Studied pH ranges were different due to dissolution of the HAp under pH 4.5 while TiO2 is stable in a wide pH range. The BRB consists of sodium compounds derived from phosphoric, boric and acetic acids – the speciation of which strongly depends on the pH value. Not only the corresponding 223Ra complexes have to be taken into account, but also the hydroxo- and carbonate-complexes especially if the pH is higher than 7 due to dissolution of atmospheric CO2.Sorption of 223Ra on superparamagnetic iron oxide nanoparticles (nFe3O4, SPIONs) was studied earlier in our laboratory,42 therefore, in the current work the results of 223Ra sorption on nHAp and nTiO2 were compared with SPIONs.
Calculation of speciation diagram for 223Ra in Britton–Robinson buffer solution
At first, it was necessary to draw attention to the fact, that the present state of stability constants knowledge for radium complexes was limited. The reliable values of studied complexes were available only for RaOH+ and RaCO3. Therefore, stability constants were taken into consideration for elements with similar chemical properties, namely for Ba, Sr and Ca. Unfortunately, data availability for Ba was also not sufficient, for Sr it was slightly better, but constants for acetic and boric acids complexes were also not available. Therefore, stability constants for Ca with above mentioned ligands were taken in addition to Ra hydroxo- and carbonate-complexes constants. The similarity of Ra and Ca complexation is demonstrated in Table 1 on the values of stability constants (β) and solubility product (SP)38,41 (the values hold for I = 0).| Compound | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/log β/log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SP SP |
|
|---|---|---|
| Ra2+ | Ca2+ | |
| CO32− | 2.50/−8.30 | 3.15/−8.14 |
| Cl− | −0.10 | −0.29 |
| SO42− | 2.75 | 2.31 |
| OH− | −13.49 | −12.78 |
The calculation of speciation diagrams were performed for the following composition of aqueous phase: 5.00 × 10−12 mol L−1 Ra(NO3)2; ∑[H3PO4] = 4.00 × 10−2 mol L−1; ∑[H3BO3] = 4.00 × 10−2 mol L−1; ∑[CH3COOH] = 4.00 × 10−2 mol L−1; pCO2 = 3.16 × 10−4 at, ionic strength I ≈ 0.15. It deals with sodium salts of above-mentioned acids in a case of higher pH values.
The values of stability and dissociation constants (I = 0) are summarized in Table 2 and the results of calculation are shown in Fig. 1. Complexing reactions and corresponding complex compounds were incorporated in the code Praspec6.fm (Table 2.). Under the given conditions, only five of them can be regarded as more important, namely, the complexing reactions with PO43−, HPO42−,H2PO4−, CO32− and (CH3COO−)2. The calculation of possible precipitation of RaCO3 was also considered in code Praspec6.fm, however, due to the very low Ra concentration, precipitation could not occur.
| Ligand | Stability constant | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
Dissociation constant | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
|---|---|---|---|---|
| a pCO2 [at] – partial pressure of CO2, atmospheric pCO2 = 3.16 × 10−4 [at], Ac = CH3COO−. | ||||
| H2PO4− | K = [CaH2PO4]/([Ca]·[H2PO4]) | 1.28 | K = [HPO4]/([H]·[PO4]) | 12.36 |
| HPO42− | K = [CaHPO4]/([Ca]·[HPO4]) | 2.68 | K = [H2PO4]/([H]2·[PO4]) | 19.70 |
| PO43− | K = [CaPO4]/([Ca]·[PO4]) | 6.46 | K = [H3PO4]/([H]3·PO4]) | 21.93 |
| H2BO3− | K = [CaH2BO3+]/([Ca]·[H2BO3−]) | 1.80 | K = [HCO3]/([H]·[CO3]) | 10.33 |
| OH− | K = [RaOH]/([Ra]·[OH]) | −0.5 | K = [H2CO3]/([H]2·[CO3]) | 16.68 |
| CO32− | K = [RaCO3]/([Ra]·[CO3]) | 2.50 | Kp = [pCO2]/([H]2·[CO3]) | 18.60a |
| Ac− | K = [CaAc+]/[Ca2+]·[Ac−] | 1.18 | K = [HAc]/([H]·[Ac]) | 4.76 |
| (Ac−)2 | K = [Ca(Ac)2]/[Ca2+]·[(Ac−)2] | 4.00 | K=([H2BO3]·[H])/[H3BO3] | −9.24 |
| (Ac−)3 | K = [Ca(Ac)3−]/[Ca2+]·[(Ac−)3] | 4.45 | K=([HBO3]·[H])/[H2BO3] | −12.74 |
| (Ac−)4 | K = [Ca(Ac)42−]/[Ca2+]·[(Ac−)4] | 3.60 | K=([BO3]·[H])/[HBO3] | −13.79 |
| — | SP = [Ra]·[CO3] | −8.30 | Kv = [H]·[OH] | −14 |
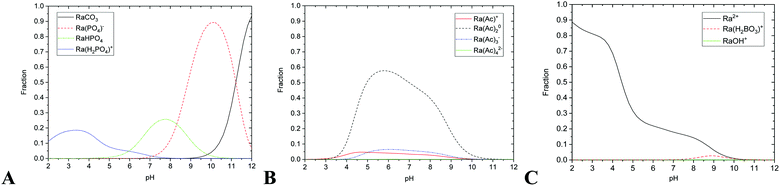 | ||
| Fig. 1 The relative abundances of studied radium species on pH (A) phosphate and carbonate complexes, (B) acetate complexes, (C) ionic radium, hydroxyl and borate complex; total ∑Ra species = 1. | ||
Modelling of experimental data as a function of pH
Chemical equilibrium model36 was used to describe the surface complexation systems. It consisted of two groups of equations. The first one characterized the protonation and ion-exchange behavior of sites (eqn (1)–(3)) and the second one the sorption of individual species including the balance equations (eqn (4)–(12)). The values of sorption constants, K, (eqn (4)–(10)) were obtained during iterations.The equations (eqn (1)–(12)) listed below were incorporated in the regression function of the code Pramg6zp.fm. The protonation reactions (eqn (1) and (2)) on edge-sites were:
| SO− + H+ ↔ SOH, KS1 = [SOH]/([SO−]·[H+]) | (1) |
| SOH + H+ ↔ SOH2+, KS2 = [SOH2+]/([SOH]·[H+]) | (2) |
The Na+/H+ ion-exchange reaction (eqn (3)) on layer-sites was:
| XNa + H+ ↔ XH + Na+, Kex = ([XH][Na+])/([XNa][H+]) | (3) |
The sorption reactions (eqn (4)–(10)) were:
| SO− + Ra2+ ↔ SORa+, K[1] = [SORa+]/([SO−]·[Ra2+]) | (4) |
| SO− + RaCO3 ↔ SORaCO3−, K[2] = [SORaCO3−]/([SO−]·[RaCO3]) | (5) |
| SO− + RaPO4− ↔ SORaPO42−, K[3] = [SORaPO42−]/([SO−]·[RaPO4−]) | (6) |
| SO− + RaHPO4 ↔ SORaHPO4−, K[4] = [SORaHPO4−]/([SO−]·[RaHPO4]) | (7) |
| 2XH + Ra2+ ↔ X2Ra + 2H+, K[5] = ([X2Ra]·[H+]2)/([XH]2·[Ra2+]) | (8) |
| XH + RaH2PO4+ ↔ XRaH2PO4 + H+, K[6] = ([XRaH2PO4]·[H+])/([XH]·[RaH2PO4+]) | (9) |
| SO− + Ra(Ac−)2 ↔ SORa(Ac)2−, K[7] = [SORa(Ac)2−]/([SO−]·[Ra(Ac)2]) | (10) |
The balance equations (eqn (11) and (12)) were:
| ∑SOH = [SO−] + [SOH2+] + [SOH0] + [SORa+] + [SORaCO3−] + [SORaPO42−] + [SORaHPO4−] + [SORa(Ac)2−] | (11) |
| ∑X = [XNa] + [XH] + 2·[X2Ra] + [XRaH2PO4] | (12) |
Following input data were also needed: the composition of liquid phase, the phase ratio V/m (L kg−1), the values of stability and dissociation constants of Ra complexes and CO2 atmospheric pressure (Table 2), protonation constants, ion-exchange constant and total concentration of edge-sites ∑SOH and layer-sites ∑X (Table 3). All data in the Table 3 were described in detail in publication Kukleva et al.35
| Constant | Units | nHAp | nTiO2 | nFe3O4 |
|---|---|---|---|---|
| Protonation constant – KS1 | [L mol−1] | 5.12 × 1011 | 2.31 × 106 | 9.65 × 108 |
| Protonation constant – KS2 | [L mol−1] | 1.19 × 105 | 1.84 × 104 | 9.13 × 105 |
| Ion-exchange constant – Kex | [—] | 3.01 × 106 | 5.67 × 107 | 3.60 × 107 |
| Concentration of edge-sites – ∑SOH | [mol kg−1] | 5.10 | 0.20 | 0.05 |
| Concentration of layer-sites – ∑X | [mol kg−1] | 0.15 | 0.67 | 0.09 |
| Specific surface area | [m2 kg−1] | 1.17 × 105 | 3.30 × 105 | 1.09 × 105 |
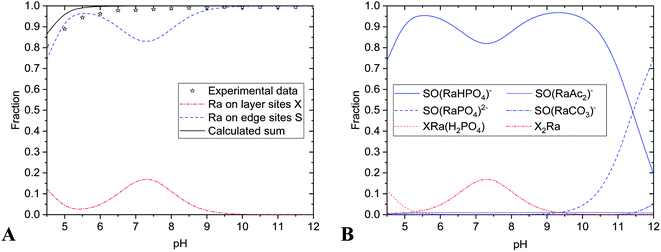
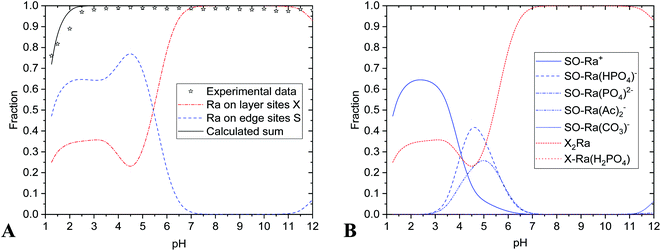
The results are summarized in Table 4 and Fig. 2 and 3. Experimental data were in a good agreement with calculated values of radium uptake as a function of pH on both edge- and layer-sites (Fig. 2A and 3A). The results of the modelling of the nHAp and nTiO2 labelling with 223Ra support the concept that the radium uptake can be described with the surface complexation model type of CEM and both edge- and layer-sites were involved in sorption mechanism (Fig. 2 and 3). It means, in our opinion, that labelling corresponds better with sorption than with co-precipitation mechanism.
| Eqn | K[—] (I = 0) | |
|---|---|---|
| nHAp | nTiO2 | |
| (4) | K[1] = 2.44 × 108 | K[1] = 4.13 × 1011 |
| (5) | K[2] = 9.87 × 104 | K[2] = 7.34 × 1011 |
| (6) | K[3] = 3.02 × 107 | K[3] = 1.69 × 1012 |
| (7) | K[4] = 3.79 × 1010 | K[4] = 6.16 × 1011 |
| (8) | K[5] = 7.85 × 10−6 | K[5] = 7.37 × 100 |
| (9) | K[6] = 6.60 × 104 | K[6] = 2.34 × 104 |
| (10) | K[7] = 2.81 × 108 | K[7] = 4.88 × 1011 |
| WSOS/DF | 0.21 | 0.18 |
The sorption efficiency (Y%) was calculated based on the equation
 | (13) |
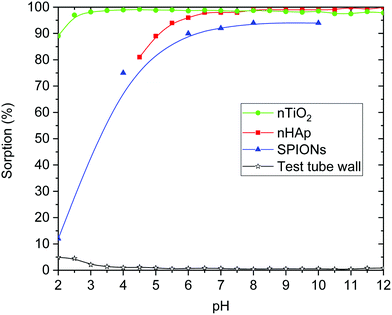
The sorption efficiency on nHAp was 95 ± 5% in the pH range from 5 to 12 (Fig. 2 and 5). It could be caused by relatively high sorption capacity or high specific surface area of nHAp (117 ± 8 m2 g−1)35 and by the composition of used aqueous solution (BRB). Fig. 2 shows that the most important sorption reactions going on edge sites were RaCO3 (eqn (4), minor), RaPO4− (eqn (6)) and RaHPO4 (eqn (7)), and on layer-sites were Ra2+ (eqn (8)) and RaH2PO4+ (eqn (9)). On the base of speciation diagrams (Fig. 1), the greater role of PO43−, CO32− and CH3COO− ligands were expected, but this supposition especially in a case of acetic anion was not confirmed. However, the role of HPO42− seems to be underestimated.
In the case of nTiO2 sorption efficiency was about 100% in pH range from 3 to 12 and decreased to 75% at lower pH values (Fig. 3 and 5), which could be also caused by high sorption capacity or high specific surface area (330 ± 10 m2 g−1)35 of nTiO2, and also due to the composition of aqueous phase. The main role in sorption reactions on layer-sites played Ra2+ itself (eqn (8) and Fig. 3). On edge-sites the most important species seems to be Ra2+ (eqn (4)), RaCO3 (eqn (5)), RaHPO4 (eqn (7)) and Ra(Ac−)2 (eqn (10)) (Fig. 4). On the base of speciation diagrams, the greater role of PO43− (Fig. 1A) and CH3COO− (Fig. 1B) ligands were expected, however this expectation were not verified. One of the most interesting result is that surface complexation model type of CEM is suitable as a describing model of studied nTiO2 radium uptake, as well as of nHAp.
Certainly, the question considering presence of atmospheric CO2 also needs to be taken into account. Relatively high concentrations of carbonates in samples with pH greater than 8–9 could affect the results, therefore, it might be better to use inert atmosphere for clearer experiments. In spite of this, the CO2 and HCO3− were included in calculations.
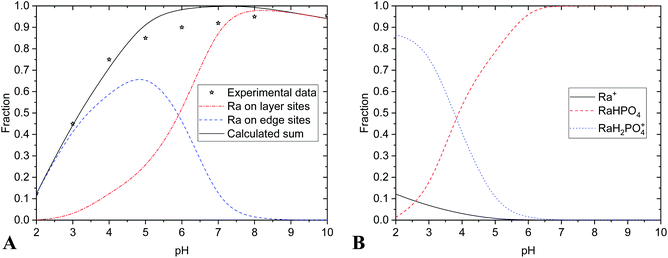
Radium sorption properties of nHAp and nTiO2 were compared also with SPIONs (Fig. 4 and 5).42 From comparison, it is evident that all three materials have relatively high sorption affinity to Ra(II) under the studied conditions. It is necessary to keep in mind that low initial radium concentrations were used (approx. 2 kBq, 5 × 10−12 M). Although there were some differences in the values of parameters of edge-sites (KS1, KS2, ∑SOH), of layer-sites (Kex, ∑X) and of the specific surface area, sorption yields for all three materials were high. Additional distinction of nHAp towards nTiO2 and SPIONs was its lower chemical stability in acidic aqueous solutions. Regarding its possible application in vivo, it should not play any significant role.
The values of nHAp's and nTiO2's KS1 (equilibrium constant of reaction eqn (1)) indicated the relatively greater shift of nHAp's protonation reaction (eqn (1)) to the right in comparison to the nTiO2. In relation to KS1 values, the deprotonated species SO− did not exist in pH lower than 6 in the case of nHAp and lower than 5 for nTiO2.35 This fact played a certain role in ion-exchange reactions (eqn (3)) and surface complexation reactions (eqn (4)–(7) and (10)).
Comparing all three materials, it could be said that all of them have comparable properties in the context of Ra sorption (Fig. 5 and 6). Hydroxyapatite nanoparticles as well as SPIONs showed high yields of 223Ra uptake at pH over 6. In the case of nHAp, it could be due to low chemical stability of nanomaterial at lower pH. In the case of SPIONs, it is probably caused by presented species (SPIONs labelling was performed in PBS, where the main component is phosphate and at pH lower than 6 it was presented in the form of H3PO4 and H2PO4− (Fig. 4)).42 Chemically stable nTiO2 has shown very high sorption yields at pH 2.5 and higher. It is also important to notice, that sorption of all presented radium species on plastic was negligible (Fig. 5).
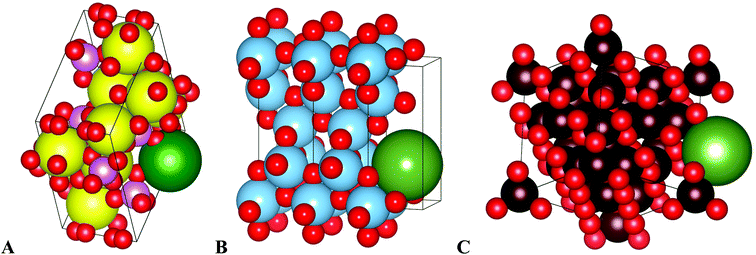 | ||
| Fig. 6 Schematical drawing of radium uptake in (A) nHAp, (B) nTiO2, (C) SPIONs. Yellow – calcium, blue – titanium, dark red – iron, green – radium, red – oxygen, pink – phosphorus. Images were created in Vesta software.43 | ||
Despite CO2 presence interfere and tangle modelling, it did not affect experimental the sorption results. This can be extremely beneficial for easier experimental setup, where necessary requirements could be lower.
Furthermore, another interesting result was that there was a good evidence for the modelling of radium uptake (labelling, sorption) on nanoparticles of nHAp, nTiO2 and SPIONs by means of the surface complexation model type of CEM, in spite of the fact, that studied materials were not similar.
Conclusions
Studied materials nHAp and nTiO2 had shown relatively high sorption affinity to Ra(II) under the studied conditions and the radiolabelling yields were over 95% in a wide pH range. Based on the calculations and modelling it was found, that the main role in sorption in the case of nHAp played RaCO3, RaPO4−, RaHPO4, Ra2+ and RaH2PO4+. In the case of nTiO2 the main role in sorption reactions played Ra2+ itself, RaCO3, RaHPO4 and Ra(Ac−)2. Furthermore, it was found, that presence of CO2 did not interfere high sorption yields, what could be important to take into account for further experiments.This paper shows possibility to use nHAp and nTiO2 nanoparticles as a useful vehicle for 223Ra delivery for targeted alpha therapy. So, it could be concluded, that nHAp and nTiO2 are suitable nanomaterials for medicinal usage due to high sorption properties in a pH range required for medicine, radiation stability and biocompatibility. Another important benefit is labelling procedure simplicity, where radium chloride in a liquid form is mixed with ready-made particles under laboratory temperature without any inert atmosphere. Obviously, this study is preliminary and further investigations of labelling kinetics and in vitro stability in biologically relevant media are necessary.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the grants of NV16-30544A (Czech Health Research Council), CZ.02.1.01/0.0/0.0/15_003/0000464 (EU and the Ministry of Education, Youth and Sports of the Czech Republic), and SGS19/194/OHK4/3T/14 (Czech Technical University in Prague).References
- U.S. National Library of Medicine, Toxicology Data Network, November 2019, Available on: https://toxnet.nlm.nih.gov/newtoxnet/index.html Search PubMed.
- M. P. Ferraz, F. J. Monteiro and C. M. Manuel, J. Appl. Biomater. Funct. Mater., 2004, 2(2), 74–80 CAS.
- S. Koutsopoulos, J. Biomed. Mater. Res., 2002, 62(4), 600–612 CrossRef CAS PubMed.
- W. Kreyling, U. Holzwarth, N. Haberl, J. Kozempel, S. Hirn, A. Wenk, C. Schleh and M. Schäffler, et al., Nanotoxicology, 2017, 11(4), 434–442 CrossRef CAS PubMed.
- M. Malekshahi Byranvand, A. Nemati Kharat, L. Fatholahi and Z. Malekshahi Beiranvand, J. Nanostruct., 2013, 3, 1–9 Search PubMed.
- S. Mital Gupta and M. Tripathi, Cent. Eur. J. Chem., 2012, 10(2), 279–294 Search PubMed.
- A. Salvador, M. C. Pascual-Martí, J. R. Adell, A. Requeni and J. G. March, J. Pharm. Biomed. Anal., 2000, 22(2), 301–306 CrossRef CAS PubMed.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46(12), 6387–6392 CAS.
- H. Maeda, K. Tsukigawa and J. Fang, Microcirculation, 2016, 23(3), 173–182 CrossRef CAS PubMed.
- J. A. Nagy, S.-H. Chang, A. M. Dvorak and H. F. Dvorak, Br. J. Cancer, 2009, 100(6), 865–869 CrossRef CAS PubMed.
- K. Van Butsele, R. Jérôme and C. Jérôme, Polymer, 2007, 48(26), 7431–7443 CrossRef CAS.
- J. Kozempel, M. Vlk, E. Málková, A. Bajzíková, J. Bárta, R. Santos-Oliveira and A. Malta Rossi, J. Radioanal. Nucl. Chem., 2014, 34(1), 443–447 CrossRef.
- L. D. Esposti, A. Tampieri and M. Iafisco, in Nanotechnologies in Preventive and Regenerative Medicine, ed. V. Uskokovic, Elsevier, New York, 2017, ch. 6.3, pp. 465–486 Search PubMed.
- M. Sakmar, M. Vlk, P. Suchankova, E. Kukleva, J. Kozempel, M. Hruby and V. Lobaz, presented in part at 13th international symposium on the synthesis and application of isotopically labelled compounds, Prague, June 2018 Search PubMed.
- P. Micolova, E. Kukleva, P. Nykl, M. Sakmar, M. Vlk, L. Nespesna and J. Kozempel, J. Labelled Compd. Radiopharm., 2017, 60(S1), S283 Search PubMed.
- S. Chakraborty, K. V. Vimalnath, A. Rajeswari, H. D. Sarma, A. Shinto, E. R. Radhakrishnan and A. Dash, J. Radioanal. Nucl. Chem., 2017, 302(2), 875–881 CrossRef.
- G. Sgouros, A. M. Ballangrud, J. G. Jurcic, M. R. McDevitt, J. L. Humm, E. Y. Erdi, B. M. Mehta, R. D. Finn, S. M. Larson and D. A. Scheinberg, J. Nucl. Med., 1999, 40(1), 1935–1946 CAS.
- W. Zhou and J. Zheng, Adv. Mater. Res., 2012, 503/504, 688–691 Search PubMed.
- J. Xie, S. Lee and X. Chen, Adv. Drug Delivery Rev., 2010, 62(11), 1064–1079 CrossRef CAS PubMed.
- C. Apostolidis, R. Molinet, J. McGinley, K. Abbas, J. Möllenbeck and A. Morgenstern, Appl. Radiat. Isot., 2005, 62(3), 383–387 CrossRef CAS PubMed.
- S. Handley-Sidhu, T. K. Mullan, Q. Grail, M. Albadarneh, T. Ohnuki and L. E. Macaskie, Sci. Rep., 2016, 6(1), 1–8 CrossRef PubMed.
- W. G. Kreyling, U. Holzwarth, C. Schleh, J. Kozempel, A. Wenk, N. Haberl, S. Hirn, M. Schäffler, J. Lipka, M. Semmler-Behnke and N. Gibson, Nanotoxicology, 2017, 11(4), 443–453 CrossRef CAS PubMed.
- G. Xie, C. Wang, J. Sun and G. Zhong, Toxicol. Lett., 2011, 205(1), 55–61 CrossRef CAS PubMed.
- E. Cedrowska, M. Pruszynski, A. Majkowska-Pilip, S. Meczyńska-Wielgosz, F. Bruchertseifer, A. Morgenstern and A. Bilewicz, J. Nanopart. Res., 2018, 20, 83, DOI:10.1007/s11051-018-4181-y.
- S. Bassot, C. Mallet and D. Stammose, MRS Online Proc. Libr., 2000, 663, 1081 CrossRef.
- M. Sajih, N. D. Bryan, F. R. Livens, D. J. Vaughan, M. Descostes, V. Phrommavanh, J. Nos and K. Morris, Geochim. Cosmochim. Acta, 2014, 146, 150–163 CrossRef CAS.
- J. M. Zachara, C. E. Cowan and C. T. Resch, Geochim. Cosmochim. Acta, 1991, 55(6), 1549–1562 CrossRef CAS.
- European Medicines Agency, Xofigo, June 2019, Available on: https://www.ema.europa.eu/en/medicines/human/EPAR/xofigo Search PubMed.
- S. Nilsson, P. Srang, A. K. Aksnes, L. Franzèn, P. Olivier, A. Pecking, J. Staffurth, S. Vasanthan, C. Andersson and Ø. S. Bruland, Eur. J. Cancer, 2012, 48(5), 678–686 CrossRef CAS PubMed.
- S. Mirzadeh, Appl. Radiat. Isot., 1998, 49(4), 345–349 CrossRef CAS.
- O. Mokhodoeva, L. Guseva and N. Dogadkin, J. Radioanal. Nucl. Chem., 2014, 304(1), 449–453 CrossRef.
- E. Kukleva, J. Kozempel, M. Vlk, P. Micolova and D. Vopalka, J. Radioanal. Nucl. Chem., 2014, 304(1), 263–266 CrossRef.
- L. I. Guseva, G. S. Tikhomirova and N. N. Dogadkin, Radiochemistry, 2004, 46(1), 58–62 CrossRef CAS.
- WWW, Table of radioactive isotopes: nuclide search, June 2019, Available on: http://nucleardata.nuclear.lu.se/toi/nucSearch.asp Search PubMed.
- E. Kukleva, P. Suchankova, K. Stamberg, M. Vlk, M. Slouf and J. Kozempel, RSC Adv., 2019, 9, 21989–21995 RSC.
- H. Filipská and K. Štamberg, Acta Polytech., 2005, 45(5), 11–18 Search PubMed.
- H. Wanner, Y. Albinsson, O. Karnland, E. Wieland, P. Wersin and L. Charlet, Radiochim. Acta, 1994, 66(67), 157–162 Search PubMed.
- ZZ HATCHES-20, Database for radiochemical modelling, May 2019, Available on: https://www.oecd-nea.org/tools/abstract/detail/nea-1210 Search PubMed.
- L. Dvořák, T. Ledvinka and M. Sobotka, FAMULUS 3.1, 1991, Custom made software, Prague, Czech Republic Search PubMed.
- A. L. Herbelin and J. C. Westall, FITEQL 3.2, Custom made software. Department of Chemistry, Oregon State University, Corvallis, Oregon, USA, 1996 Search PubMed.
- ThermoChimie, Thermodynamic database, August 2019, Available on: https://www.thermochimie-tdb.com Search PubMed.
- O. Mokhodoeva, M. Vlk, E. Málková, E. Kukleva, P. Mičolová, K. Štamberg, M. Šlouf, R. Dzhenloda and J. Kozempel, J. Nanopart. Res., 2016, 18(10), 1–12 CrossRef CAS.
- K. Momma, August 2019, Available on: https://jp-minerals.org/vesta/en/.
| This journal is © The Royal Society of Chemistry 2020 |