Open Access Article
Open Access ArticlePhysicochemical analysis and phenolic profile of polyfloral and honeydew honey from Montenegro†
Milica Nešovića,
Uroš Gašić b,
Tomislav Tostic,
Jelena Trifkovićc,
Rada Baošićc,
Stevan Blagojevića,
Ljubiša Ignjatovićd and
Živoslav Tešić
b,
Tomislav Tostic,
Jelena Trifkovićc,
Rada Baošićc,
Stevan Blagojevića,
Ljubiša Ignjatovićd and
Živoslav Tešić *c
*c
aInstitute of General and Physical Chemistry, Studentski trg 12-16, 11158 Belgrade, Serbia
bInstitute for Biological Research “Siniša Stanković”, National Institute of Republic of Serbia, University of Belgrade, Bulevar despota Stefana 142, 11060 Belgrade, Serbia
cFaculty of Chemistry, University of Belgrade, Studentski trg 12-16, 11158 Belgrade, Serbia. E-mail: ztesic@chem.bg.ac.rs
dFaculty of Physical Chemistry, University of Belgrade, Studentski trg 12-16, 11158 Belgrade, Serbia
First published on 14th January 2020
Abstract
The research subject of this paper was a detail physicochemical analysis of 28 honey samples from the northern part of Montenegro. The honey from Montenegro has not been previously studied in such detail. Differentiation between samples, such as honeydew honey and polyfloral honey, was based on electrical conductivity, which was higher than 0.8 mS cm−1 for honeydew honey, as was expected. Other investigated physicochemical parameters (water content, free acids, diastase activity, hydroxymethylfurfural (HMF) content and sugar content) have shown great similarity for all honey samples. The main interest of this study was the identification and quantification of phenolic compounds using ultra-high performance liquid chromatography (UHPLC) with mass spectrometry detection. The results show that honey samples are very rich in phenolic compounds, especially quercetin. Among the 31 quantified phenolic compounds, the most dominant were phenolic acids. The highlight was based on p-hydroxybenzoic acid, p-coumaric acid, caffeic acid and ferulic acid. Considering polyphenolic compounds and sugar content, a high nutritional value can be observed in all samples, with an emphasis on polyfloral honeys, as was confirmed with principal component analysis (PCA). In addition, all honey samples were tested for total phenolic content (TPC) and radical scavenging activity (RSA). The results indicate the higher antioxidant ability of honeys from Montenegro in comparison to some honey samples from other countries in the region.
Introduction
Honeybee products are very popular and highly valued food products with a widespread use in nutrition and medicine. They have a potential role in contributing to human health. Honey is a highly energetic sweet food. It is an easily accessible source of energy. European Legislation1 has provided a definition of honey. Based on that, bees produce a floral honey from the collected nectar, and honeydew honey from collected sugar rich excretion of plants or excrement of insects. Which honey they produce primarily depends on their location and geographical area that will affect the availability of food source. Polyfloral honey originates from flowers of blossoming plants. Honeydew honey, also called forest honey, made from honeydew, is mainly found on plants such as conifers (fir, pine, spruce), but also oak, beech, etc. Nectar and honeydew may contain different phenolic compounds and sugars, which leads to the diverse characteristics of each type of honey. Some studies have shown appreciably higher content of bioactive compounds for honeydew honey that causes its higher antioxidant activity.2–5 These higher values point to more therapeutic properties of honeydew honey, which give a rise to its commercial interest. The demand for honeydew honey is increasing, especially in certain regions of Europe where consumers enjoy its remarkable flavor.6,7 Honeydew honey usually has lower content of monosaccharides and higher content of di- and trisaccharides, such as melezitose, maltotriose or raffinose, which is a good indicator for the recognition of honeydew type of honey.6Montenegro is a country in the southwestern part of the Balkan. For a relatively small area, it has rich biodiversity due to its geographic location and nearness of the sea. Northern part of Montenegro is the area of mountains and valleys. Water wealth and numerous meadows and pastures give contribute to the abundance of significant honey plants. The areas of the mountains include diverse forest vegetation through which permeates silicate rocks and limestone. Mostly dominant forests in high mountains areas are spruce forests, in the lower there are also fir trees, and in the valleys of river oak forests. In the basin of Bijelo Polje and Berane, there are also beech forests. This contributes to its rich flora and excellent natural conditions for beekeeping. The distribution of vegetation at different altitudes is important due to flowering and enables extension of the bee pasture. Thanks to the floral diversity and successive bloom of good melliferous plants, bees use meadow pasture for a long-period time. However, at the end of summer and in early autumn, the contribution of forest bee pasture becomes important because of the honeydew.
Until now, different techniques of honey characterization are well known developed in the literature, and a selection of the most appreciated, such as liquid chromatography (LC), is presented in this study.
This study presents a physicochemical analysis of honey samples from northern part of Montenegro. The main goal was a detailed characterization of these honey samples, which had not been thoroughly examined before. This study is focused on three issues: (1) physicochemical analysis, (2) phenolic profile analysis and evaluating of its influence on antioxidant activity, and (3) an attempt to group the obtained results in order to distinguish two types of honeys. The first topic present the physicochemical characterization of honey samples, which relays on determination of water content, electrochemical conductivity, free acidity, HMF content, and sugar content, as a factors of general classification and descriptions of honey.8 A very important issue of potential establishing sample differentiation was given to the measurement of electrochemical conductivity. The sugar profile was determined by using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Particular topic include identification and quantification of polyphenolic compounds, using the UHPLC system coupled with linear trap quadrupole and OrbiTrap mass analyzer (UHPLC-LTQ OrbiTrap MS) and UHPLC with a diode array detector and a triple quadrupole mass spectrometer (UHPLC-DAD-MS/MS). Spectrophotometric test, such as TPC and RSA was also observed. Chemometric technique PCA, which was performed on all determined parameters, was used to distinguish obtained honey samples according to their origin. In addition, a summary outlining of the obtained results was compared with the results of other authors that are found in the literature.
Experimental
Honey samples
“The Association of the Beekeeping Organizations of Montenegro” provided twenty-eight honey samples, marked as a polyfloral honey. Honey samples were collected during August–September 2017 from three locations: Berane (42.84°N 19.86°E), Bijelo Polje (43.04°N 19.75°E), and Pljevlja (43.36°N 19.36°E), which are in the northern part of Montenegro.Reagents, standards and materials
All chemicals (sodium hydroxide, starch, acetate buffer, sodium chloride, 5-hydroxymethyl-furan-2-carbaldehyde, Folin–Ciocalteu's reagent, sodium carbonate, gallic acid, methanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrochloric acid, acetonitrile) were of analytical grade. Stock solutions, diluted solutions and blank samples were prepared with ultrapure water 18 MΩcm (0.055 μS cm−1) resistivity obtained from a TKA MicroPure water purification system (Thermo Fisher, TKA, Germany). Sugar standards (glucose, fructose, sucrose, maltose, isomaltose, trehalose, turanose, melibiose and melezitose) were purchased from Tokyo Chemical Industry, Europe (Zwijndrecht, Belgium). Standards of phenolic compounds were purchased from Sigma-Aldrich (Steinheim, Germany). Syringe filters (25 mm, nylon membrane, 0.45 μm) were from Supelco (Bellefonte, PA). Cartridges for solid phase extraction (SPE) used for preconcentration of samples were Strata C18-E (500 mg/3 mL) obtained from Phenomenex (Torrance, USA).Physicochemical parameters were determined according to the method described by Bogdanov et al.8 Water content was determined by measuring the refractive index of honey samples at the temperature of 20 °C using an Abbe-type refractometer (Atago RX refractometer, Japan) and Chataway's table correction. Electrical conductivity of a 20% (w/v) aqueous solution of honey was measured using a conductivity meter (WTW Golden lab & engineering, Cond 7110, Inolab) equipped with conductivity cell with the constant of 0.108 cm−1. Free acidity was determined by titration of the honey solutions (5 g of honey were dissolved in 40 mL of ultrapure water) with sodium hydroxide (0.05 M) to pH 8.5, followed by pH-metry. Determination of diastase activity was based on hydrolysis of 1% starch solution by an enzyme from honey at a temperature of 40 °C, as was described by Bogdanov et al.8 The absorbance of the honey solutions was measured at different time intervals on a wavelength of 660 nm using the UV/Vis spectrophotometer (GBC UV/Visible Cintra 6, Australia). Results were expressed in Schade units per gram of honey and termed as diastase number (DN). Content of HMF was determined using the HPLC system with UV detection (Waters 1525 HPLC dual pump system).8 The preparation of honey samples and measurement of sugar content was carried out by using a HPAEC/PAD method as exactly as described by Gašić et al.9
Phenolic profile
The extraction of phenolic compounds from honey samples was carried out by using as exactly as described by Gašić et al.10Qualitative analysis of phenolic compounds was performed by UHPLC system with a quaternary Accela 600 pump and Accela autosampler (Thermo Fisher Scientific, Bremen, Germany) connected to LTQ OrbiTrap MS with heated electrospray ionization probe (HESI-II, Thermo Fisher Scientific, Bremen, Germany). Analytical column Syncronis C18 column (100 × 2.1 mm, 1.7 μm particle size; Thermo Fisher Scientific) was used for separation of phenolic compounds. The chromatographic conditions were previously described by Gašić et al.10 For qualitative analysis of phenolic compounds six honey samples were chosen, one honeydew honey and one polyfloral honey sample from each area.
UHPLC equipped with a diode array detector (DAD) connected to TSQ Quantum Access Max triple-quadrupole mass spectrometer (Thermo Fisher Scientific, Basel, Switzerland) was used for quantitative analysis of phenolic compounds. The separation of phenolic compounds was achieved by using a Syncronis C18 column (100 × 2.1 mm, 1.7 μm). Chromatographic conditions and mass spectrometry parameters for quantification of phenolic compounds were previously described by Gašić et al.9
Determination of total phenolic content (TPC) and radical scavenging activity (RSA)
For TPC and RSA determination, honey samples were prepared according to method proposed by Gašić et al.10TPC was evaluated by method described by Meda et al.11 The absorbance was measured at 765 nm by UV/Vis spectrophotometer (GBC UV/Visible Cintra6, Australia).
RSA was determined by method published by Chua et al.,12 with some modifications. Prepared honey extracts (0.1 g mL−1) in 4 mL of DPPH methanol solution were left in the dark for 60 minutes for incubation.
Data analysis
Descriptive statistics and Mann–Whitney test were performed by SPSS statistical software (IBM SPSS Statistics 20, IBM Corporation). Principal component analysis has been carried out by PLS ToolBox, v.6.2.1, for MATLAB 7.12.0 (R2011a). All data were autoscaled prior to any multivariate analysis. PCA was carried out by using a singular value decomposition algorithm and a 0.95 confidence level for Q and T2 Hotelling limits for outliers.Results and discussion
Honey classification
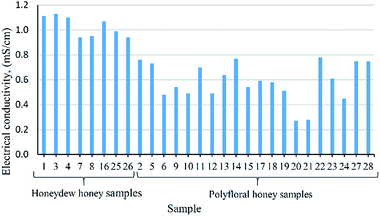
Obtained honey samples were specified as polyfloral, only by beekeepers from the “The Association of the Beekeeping Organizations of Montenegro”. Through analysis, the honey samples, have been differently identified as polyfloral and honeydew honeys. Based on the electrical conductivity of the 28 honey samples, eight samples belong to the group of honeydew honey, marked as No. 1, 3, 4, 7, 8, 16, 25, 26 (Fig. 1). This classification is a consequence of their higher values of electrical conductivity, above 0.8 mS cm−1, which is the limit value for honeydew honey.1 There are studies that also reported this way of honey classification.3,6,13,14Physicochemical analysis
The results of descriptive analysis for physicochemical parameters of 28 honey samples, their main values, standard deviations and ranges are presented in Table 1, while the results obtained for each sample are shown in Table S1.† The determined values of water content are below than 17.15% (Table 1) and they are in accordance to international requirement with levels lower than 20%.1 These values point to an appropriate storage and beekeepers handling of the honey. Similar values of low water content in polyfloral and/or honeydew honey samples were also reported by other authors.14–17 The electrical conductivity of these samples is the cause of the classification between them. The obtained values for honeydew honey samples varied between 0.94–1.13 mS cm−1 (Table 1). These results are similar with reported values for some honeydew honey samples from other geographical origins such as Romania,16 Serbia,18 Bulgaria,19 Croatia,20 Brazil,21 or with fir honeydew honey from Greece.22 Range of electrical conductivity for polyfloral honey goes from 0.27 to 0.78 mS cm−1, very closely to the range 0.40–0.75 reported by Nascimento et al.23 The mean value for polyfloral honey is 0.59 mS cm−1, similar to the results of Popek et al.14 Results of free acidity is in compliance with recommended value of not more than 50 meq. kg−1 of honey.1 Mean value for free acidity in honeydew honey are higher than in polyfloral honey (Table 1). The diastase activity of tested honey samples is higher than 8 Schade units, which is the minimal value recommended by the EU Directive 2014/63.1 Results of the content of HMF in honey samples are mostly below the detection limit, <5 mg kg−1, except in six samples (Table S1†), but still in permitted range, up to 40 mg kg−1.1 Higher content of HMF was noticed in polyfloral honey samples (Table 1).| Variable | Honeydew honey | Polyfloral honey | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min–max | Mean | SD | Median | Min–max | |
| a Mean – mean value, SD – standard deviation, median – median value, min–max – concentration range. | ||||||||
| Water content (%) | 15.96 | 0.77 | 16.28 | 14.93–16.88 | 16.08 | 0.67 | 15.98 | 14.85–17.15 |
| Electrical conductivity (mS cm−1) | 1.03 | 0.08 | 1.03 | 0.94–1.13 | 0.59 | 0.15 | 0.59 | 0.27–0.78 |
| Free acidity (meq. kg−1) | 29.84 | 3.66 | 29.05 | 25.81–36.63 | 27.18 | 5.08 | 28.38 | 17.41–34.37 |
| Diastase activity (DN) | 31.64 | 2.34 | 31.85 | 27.93–34.81 | 34.14 | 6.14 | 33.58 | 19.77–46.11 |
| HMF (mg kg−1) | 0.69 | 1.94 | 0 | 0–5.50 | 2.33 | 4.24 | 0 | 0–10.95 |
| Glucose (g/100 g) | 29.48 | 2.83 | 29.59 | 24.98–34.19 | 30.56 | 2.87 | 30.37 | 26.41–36.08 |
| Fructose (g/100 g) | 36.35 | 2.72 | 36.77 | 32.58–41.41 | 36.21 | 2.89 | 35.67 | 31.45–41.27 |
| Sucrose (g/100 g) | 1.62 | 0.94 | 1.33 | 0.43–3.01 | 1.43 | 0.78 | 1.09 | 0.51–3.35 |
| Maltose (g/100 g) | 0.74 | 0.18 | 0.74 | 0.54–1.00 | 1.09 | 0.59 | 0.86 | 0.30–2.57 |
| Isomaltose (g/100 g) | 0.49 | 0.19 | 0.53 | 0.22–0.79 | 0.68 | 0.34 | 0.59 | 0.24–1.47 |
| Trehalose (g/100 g) | 0.52 | 0.35 | 0.43 | 0.15–1.08 | 0.29 | 0.21 | 0.27 | 0.02–0.72 |
| Turanose (g/100 g) | 0.80 | 0.16 | 0.79 | 0.60–1.07 | 0.65 | 0.55 | 0.48 | 0.08–2.14 |
| Melibiose (g/100 g) | 0.05 | 0.03 | 0.06 | 0–0.08 | 0.06 | 0.07 | 0.03 | 0–0.27 |
| Melezitose (g/100 g) | 0.27 | 0.24 | 0.22 | 0.03–0.59 | 0.15 | 0.14 | 0.11 | 0.001–0.53 |
| Sum of sugars (g/100 g) | 70.33 | 6.45 | 70.69 | 62.64–79.91 | 71.13 | 7.49 | 69.30 | 62.19–85.03 |
The results of descriptive analysis of sugar content in honey samples are presented in Table 1. Content of monosaccharides is 65.84 g/100 g for honeydew honey and 66.77 g/100 g for polyfloral honey. The determined reducing monosaccharides, glucose and fructose, which are the major constituents of honey, are in agreement with the proposed value by European Legislation.1 There is a similarity in determined range of fructose and glucose content for both types (Table 1). Salonen et al.24 also reported no significant differences of monosaccharides between polyfloral and honeydew honey samples. The content of sucrose in all samples is less than the maximum allowed concentration of 5 g/100 g.1 The trisaccharide melezitose was also evidenced in all samples (Fig. S1†) containing higher main value in honeydew honey (0.27 g/100 g) than in polyfloral honey samples (0.15 g/100 g) (Table 1). Melezitose is recognized as a good indicator in differentiation honeydew honey from polyfloral honey.6
According to the results of the physicochemical parameters, all samples are within the established limits and in agreement with international quality regulations, suggesting good conditions of honey production. As other studies shown,6,14,21 physicochemical variables were successfully used to differentiate honeydew honey from floral honey, but in our study, specific electrical conductivity appears to be the most effective in distinguishing these two types of honey. Moreover, botanical origin of honey could be successfully determined by electrical conductivity, among the other physicochemical parameters.3,13 Considering the results of sugar content, only negligible extent content of melezitose indicate differences between samples, while other studies suggested considerable lower levels of monosaccharides,13 and higher levels of oligosaccharides6,7 in honeydew honey. Great similarity of physicochemical parameters for two types of honey types originating from a small geographical region indicates that honeydew honeys and polyfloral honeys represent a complex mixture of honeydew and nectar from different botanical species.
Phenolic profile
| No | Compound name | tR, min | Molecular formula, [M − H]− | Calculated mass, [M − H]− | Exact mass, [M − H]− | Δ ppm | MS2 fragments, (% base peak) | MS3 fragments, (% base peak) | Berane | Bijelo Polje | Pljevlja | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H4 | P6 | H16 | P9 | H25 | P20 | |||||||||
| a Confirmed using available standards. | ||||||||||||||
| Phenolic acids and derivatives | ||||||||||||||
| 1 | Protocatechuic acida | 4.53 | C7H5O4− | 153.0193 | 153.0191 | 1.57 | 109(100), 95(75), 79(20), 59(10) | 81(100), 68(25), 65(15) | + | + | + | + | + | + |
| 2 | 3-O-Caffeoylquinic acid | 5.37 | C16H17O9− | 353.0878 | 353.0872 | 1.76 | 191(100), 179(30), 135(10) | 173(75), 127(100), 111(40), 93(60), 85(90) | − | + | − | − | − | − |
| 3 | p-Hydroxybenzoic acida | 5.54 | C7H5O3− | 137.0244 | 137.0242 | 1.39 | 109(10), 93(100) | 93(100) | + | + | + | + | + | + |
| 4 | 5-O-Caffeoylquinic acida | 5.86 | C16H17O9− | 353.0878 | 353.0875 | 0.82 | 191(100), 179(5) | 173(75), 127(100), 111(40), 93(60), 85(90) | − | + | − | − | − | − |
| 5 | Caffeic acida | 5.92 | C9H7O4− | 179.0350 | 179.0345 | 2.85 | 135(100) | 135(60), 117(15), 107(100), 91(55), 79(15) | + | + | + | + | + | + |
| 6 | p-Coumaric acida | 6.81 | C9H7O3− | 163.0401 | 163.0398 | 1.41 | 119(100) | 119(60), 101(20), 93(25), 91(100), 72(10) | + | + | + | + | + | + |
| 7 | Ellagic acid | 6.83 | C14H5O8− | 300.9990 | 300.0996 | −2.13 | 284(40), 271(60), 257(100), 229(85), 185(40) | 229(100), 213(20), 185(85) | + | + | − | + | − | − |
| 8 | Dihydroxybenzoic acid methyl ether | 6.97 | C8H7O4− | 167.0350 | 167.0346 | 2.51 | 152(100), 108(20) | 108(100) | + | − | − | − | − | − |
| 9 | Benzyl caffeate | 11.56 | C16H13O4− | 269.0819 | 269.0815 | 1.45 | 225(10), 179(10), 178(70), 161(10), 134(100) | 121(90), 111(40), 106(100) | + | + | + | + | + | + |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Flavonoids and derivatives | ||||||||||||||
| 10 | Tricetin | 6.58 | C15H9O7− | 301.0354 | 301.0353 | 0.10 | 283(100), 273(50), 255(15), 245(20), 227(15) | 255(100), 239(70), 227(70), 211(40), 199(20) | − | − | + | + | − | + |
| 11 | Quercetin 3-O-rhamnosidea | 7.30 | C21H19O11− | 447.0933 | 447.0927 | 1.36 | 301(100), 300(35), 284(20) | 273(25), 257(20), 179(100), 151(75) | + | + | + | + | + | + |
| 12 | Kaempferol 3-O-rhamnoside | 7.77 | C21H19O10− | 431.0984 | 431.0974 | 2.37 | 327(5), 285(100), 284(70), 255(10) | 267(50), 257(100), 241(40), 229(60), 213(30) | + | + | − | + | + | + |
| 13 | Kaempferol 7-O-rhamnoside | 8.56 | C21H19O10− | 431.0984 | 431.0972 | 2.78 | 286(10), 285(100), 284(20), 257(5), 151(5) | 257(40), 241(30), 213(10), 151(100), 107(10) | − | + | + | + | + | + |
| 14 | Luteolina | 8.80 | C15H9O6− | 285.0405 | 285.0403 | 0.63 | 257(40), 241(100), 217(50), 199(70), 175(70) | 255(50), 227(100), 211(75), 197(35), 183(85) | + | + | + | + | + | + |
| 15 | Quercetina | 8.87 | C15H9O7− | 301.0354 | 301.0345 | 3.06 | 283(15), 271(60), 257(25), 179(100), 151(80) | 151(100) | + | + | + | + | + | + |
| 16 | Pinobanksin 5-methyl ether | 9.18 | C16H13O5− | 285.0771 | 285.0761 | 1.30 | 267(100), 252(20), 239(30), 179(5), 165(5) | 252(100), 239(5), 224(10) | + | + | + | + | − | − |
| 17 | Naringenina | 9.66 | C15H11O5− | 271.0612 | 271.0599 | 4.76 | 225(5), 177(10), 151(100) | 107(100) | + | + | + | + | + | + |
| 18 | Apigenina | 9.67 | C15H9O5− | 269.0455 | 269.0456 | −0.30 | 225(5), 177(15), 151(100) | 65(100) | + | + | + | + | + | + |
| 19 | Kaempferola | 9.83 | C15H9O6− | 285.0405 | 285.0398 | 2.35 | 255(100), 227(10) | 211(100), 195(5), 167(15) | + | + | + | + | + | + |
| 20 | Methoxy kaempferol | 9.84 | C16H11O7− | 315.0510 | 315.0496 | 4.57 | 301(20), 300(100) | 272(100), 256(60), 216(20), 202(20), 166(35) | + | + | + | + | + | + |
| 21 | Pinobanksin | 9.98 | C15H11O5− | 271.0612 | 271.0600 | 4.43 | 253(100), 225(29), 269(21), 215(16), 197(15) | 197(100), 225(85), 209(51), 185(23) | + | + | + | + | + | + |
| 22 | Isorhamnetin | 10.06 | C16H11O7− | 315.0510 | 315.0506 | 1.27 | 301(20), 300(100) | 283(30), 271(100), 255(50), 227(52), 151(90) | + | + | + | + | + | + |
| 23 | Chrysoeriol | 10.16 | C16H11O6− | 299.0561 | 299.0555 | 1.91 | 285(10), 284(100) | 255(100), 227(100) | + | + | + | + | + | + |
| 24 | Quercetin dimethyl ether isomer 1 | 10.33 | C17H13O7− | 329.0667 | 329.0672 | −1.67 | 314(100) | 299(100), 271(10) | + | + | + | + | + | + |
| 25 | Rhamnetin | 10.85 | C16H11O7− | 315.0510 | 315.0512 | −0.57 | 300(40), 207(10), 193(40), 165(100), 121(10) | 150(50), 121(100), 97(60), 91(15), 65(20) | + | + | + | + | + | + |
| 26 | Quercetin dimethyl ether isomer 2 | 11.22 | C17H13O7− | 329.0667 | 329.067 | −0.94 | 314(100) | 299(100), 271(10) | + | + | + | + | + | + |
| 27 | Chrysina | 11.76 | C15H9O4− | 253.0506 | 253.050 | 3.56 | 253(30), 209(100), 181(20), 165(15), 151(15) | 181(100), 165(30), 153(20), 141(10) | + | + | + | + | + | + |
| 28 | Sakuranetin | 11.81 | C16H13O5− | 285.0769 | 285.076 | 2.07 | 270(40), 268(100), 243(50), 164(50), 151(10) | 240(100), 239(40), 223(30), 207(20), 193(10) | + | + | + | + | + | + |
| 29 | Pinocembrina | 11.89 | C15H11O4− | 255.0663 | 255.066 | 2.86 | 213(100), 187(15), 151(30), 145(10), 107(5) | 185(100), 169(20), 145(20) | + | + | + | + | + | + |
| 30 | Galangina | 12.01 | C15H9O5− | 269.0455 | 269.045 | 0.93 | 241(40), 227(80), 213(100), 197(90), 169(50) | 211(10), 198(20), 185(40), 169(100), 143(25) | + | + | + | + | + | + |
| 31 | Kaempferidea | 12.05 | C16H11O6− | 299.0561 | 299.056 | 0.47 | 284(100), 271(20), 255(5), 240(5), 165(20) | 255(25), 240(25), 227(20), 164(40), 151(100) | + | + | + | + | + | + |
| 32 | Acacetina | 12.41 | C16H11O5− | 283.0612 | 283.061 | −0.53 | 268(100) | 268(100), 240(30) | + | + | + | + | + | + |
| Phenolic compounds | Honeydew | Polyfloral | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min–max | Mean | SD | Median | Min–max | |
| a Mean – mean value, SD – standard deviation, median – median value, min–max – concentration range. | ||||||||
| Flavonoids | ||||||||
| Quercetin | 2.709 | 1.305 | 2.795 | 1.137–4.947 | 3.038 | 2.348 | 2.398 | 0.469–9.865 |
| Kaempferol | 0.901 | 0.253 | 0.836 | 0.573–1.322 | 0.961 | 0.396 | 0.974 | 0.196–1.886 |
| Galangin | 0.747 | 0.646 | 0.519 | 0.130–2.059 | 0.858 | 0.599 | 0.702 | 0.056–1.820 |
| Kaempferide | 0.044 | 0.038 | 0.031 | 0.007–0.121 | 0.042 | 0.028 | 0.036 | 0.008–0.133 |
| Apigenin | 0.710 | 0.336 | 0.739 | 0.280–1.323 | 0.939 | 0.43 | 1.038 | 0.138–1.676 |
| Chrysin | 1.609 | 1.031 | 1.323 | 0.395–3.440 | 2.166 | 1.289 | 1.974 | 0.252–4.515 |
| Acacetin | 0.043 | 0.015 | 0.045 | 0.017–0.059 | 0.050 | 0.017 | 0.050 | 0.011–0.082 |
| Luteolin | 0.096 | 0.030 | 0.092 | 0.058–0.142 | 0.172 | 0.12 | 0.149 | 0.015–0.551 |
| Genkwanin | 0.054 | 0.020 | 0.058 | 0.021–0.078 | 0.068 | 0.027 | 0.065 | 0.014–0.121 |
| Pinocembrin | 1.289 | 1.181 | 0.915 | 0.314–3.954 | 1.448 | 1.123 | 1.206 | 0.055–3.843 |
| Naringenin | 0.234 | 0.166 | 0.244 | 0.017–0.475 | 0.203 | 0.169 | 0.157 | 0.033–0.692 |
| Eriodictyol | 0.018 | 0.030 | 0.009 | 0–0.088 | 0.022 | 0.044 | 0.018 | 0–0.201 |
| Taxifolin | 0.061 | 0.061 | 0.058 | 0–0.148 | 0.043 | 0.050 | 0.014 | 0–0.154 |
| Daidzein | 0.016 | 0.024 | 0.003 | 0–0.064 | 0.027 | 0.053 | 0.007 | 0–0.200 |
| Sum of flavonoids | 8.533 | 3.857 | 7.113 | 5.047–15.371 | 9.872 | 4.911 | 8.663 | 1.637–21.286 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Phenolic acids | ||||||||
| p-Hydroxybenzoic acid | 2.182 | 1.081 | 1.667 | 1.374–4.475 | 2.318 | 0.821 | 2.315 | 0.679–3.621 |
| Protocatechuic acid | 1.008 | 0.338 | 0.990 | 0.618–1.529 | 0.721 | 0.527 | 0.705 | 0.046–1.858 |
| Vanillic acid | 1.319 | 0.347 | 1.318 | 0.871–1.892 | 1.093 | 0.275 | 1.052 | 0.509–1.841 |
| p-Hydroxyphenylacetic acid | 0.946 | 0.318 | 0.868 | 0.670–1.693 | 0.895 | 0.454 | 0.788 | 0.461–2.306 |
| Caffeic acid | 2.125 | 0.956 | 2.066 | 1.021–4.220 | 2.697 | 1.575 | 2.078 | 0.748–6.707 |
| 5-O-Caffeoylquinic acid | 0 | 0 | 0 | 0 | 1.154 | 0.511 | 0 | 0–2.229 |
| p-Coumaric acid | 2.802 | 1.194 | 2.480 | 1.671–4.699 | 2.949 | 1.167 | 3.020 | 0.895–5.416 |
| Ferulic acid | 2.077 | 0.983 | 1.903 | 0.777–3.411 | 2.554 | 1.221 | 2.445 | 0.703–4.929 |
| Sinapic acid | 0.033 | 0.066 | 0 | 0–0.177 | 0.022 | 0.047 | 0 | 0–0.148 |
| Sum of phenolic acids | 12.327 | 4.206 | 11.276 | 7.022–18.566 | 13.402 | 3.823 | 12.759 | 6.153–20.338 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Glycosides | ||||||||
| Apigenin-7-O-glucoside (apigetrin) | 0.001 | 0.002 | 0 | 0–0.005 | 0.004 | 0.007 | 0 | 0–0.021 |
| Apigenin-8-C-glucoside (vitexin) | 0.001 | 0.002 | 0 | 0–0.007 | 0.002 | 0.004 | 0 | 0–0.012 |
| Kaempferol-7-O-glucoside | 0.023 | 0.026 | 0.021 | 0–0.078 | 0.056 | 0.044 | 0.045 | 0–0.171 |
| Isorhamnetin-3-O-glucoside | 0.004 | 0.011 | 0 | 0–0.031 | 0.003 | 0.127 | 0 | 0–0.057 |
| Aesculetin-6-β-D-glucoside (aesculin) | 0.005 | 0.015 | 0 | 0–0.041 | 0.008 | 0.018 | 0 | 0–0.056 |
| Naringenin-7-O-glucoside (naringin) | 0.121 | 0.042 | 0.109 | 0.086–0.208 | 0.205 | 0.183 | 0.170 | 0–0.805 |
| Quercetin-3-O-rutinoside (rutin) | 0.050 | 0.045 | 0.049 | 0–0.142 | 0.069 | 0.064 | 0.074 | 0–0.201 |
| Quercetin-3-O-galactoside (hyperoside) | 0.016 | 0.007 | 0.014 | 0.006–0.026 | 0.026 | 0.012 | 0.024 | 0–0.052 |
| Quercetin-3-O-rhamnoside | 0.148 | 0.129 | 0.111 | 0–0.373 | 0.133 | 0.130 | 0.099 | 0–0.586 |
| Sum of glycosides | 0.369 | 0.145 | 0.362 | 0.210–0.622 | 0.505 | 0.241 | 0.485 | 0.246–1.206 |
| Sum of phenolic compounds | 21.229 | 6.875 | 20.260 | 13.091–33.798 | 23.780 | 7.911 | 23.651 | 8.046–38.363 |
| TPC | 72.884 | 9.106 | 71.673 | 60.448–92.083 | 70.019 | 22.491 | 63.828 | 39.163–110.645 |
| RSA | 10.008 | 2.803 | 8.898 | 8.151–16.521 | 8.155 | 4.826 | 7.459 | 2.361–21.273 |
The main health benefits of polyphenols include mechanisms of anti-oxidative and anti-free radical effects. Phenolic acids, as free radical scavengers, have pronounced antioxidant activity. In our study, phenolic acids have the highest proportion in total phenolic compounds. Among nine quantified phenolic acids, six phenolic acids are present in all studied samples. Four of them (p-coumaric acid, caffeic acid, p-hydroxhybenzoic acid, and ferulic acid) contain higher mean values in polyfloral samples, and two acids (protocatechuic acid and p-hydrohyphenylacetic acid) have higher mean values in honeydew honey samples (Table 3). After the quercetin, p-coumaric acid has the next dominant proportion in total phenolic compounds, followed by caffeic acid, ferulic acid, and p-hydroxybenzoic acid. Maximum value of p-coumaric acid for polyfloral samples was 5.416 mg kg−1, and for honeydew honey samples 4.699 mg kg−1 (Table 3). In contrary, Gašić et al.10 reported higher results such as maximum value of 9.97 mg kg−1 for polyfloral sample from East Serbia, and Oroian et al.16 reported 9.1 mg kg−1 for polyfloral and 8.4 mg kg−1 for honeydew honey samples from East Romania. One of the most common phenolic acids is caffeic acid. It has two hydroxyl groups bonded to benzene ring, which directly influence on its antioxidant activity.28 Its range in honey samples goes from 0.748 to 6.707 mg kg−1 (Table 3), which is similar to results of Holouzka et al.,26 but one size higher than Ciucure et al.5 and Socha et al.28 were reported. No occurrence of 5-O-caffeoylquinic acid in honeydew honey (Fig. S2†) is in contrast to results published by Vasić et al.20 who showed its presences in all honeydew honey samples from Croatia in range from 0.004 to 0.082 mg kg−1. Hydroxybenzoic acid and its derivatives have ability to modify cellular signalling processes that introduces a multiplier effect on enhancement of multiple anti-oxidant mechanisms.31 In this study, the highest value of p-hydroxybenzoic acid is 4.475 mg kg−1 in honeydew honey sample. In flavonoids native forms, they are found as both O- and C-glycosides, with O-glycosides being more common and usually better absorbed. Occurrence of glycosides considerably fluctuated going through the samples (Fig. S2†). Nine flavonoid-glycosides were identified. Naringenin-7-O-glucoside (naringin) and quercetin-3-O-rhamnoside are dominant, and then quercetin-3-O-rutinoside (rutin). Apigenin-7-O-glucoside, apigenin-8-C-glucoside and aesculetin-6-β-D-glucoside were present only in one honeydew honey sample, while isorhamnetin-3-O-glucoside is present only in one polyfloral and one honeydew honey sample.
It can be noticed that in polyfloral honey samples, is present higher average values of sums of flavonoids, phenolic acids and glycosides (9.87 mg kg−1, 13.40 mg kg−1, and 0.51 mg kg−1, respectively) than in honeydew honey samples (8.53 mg kg−1, 12.33 mg kg−1, and 0.37 mg kg−1, respectively) (Table 3). Opposite to these values, Ciucure et al.5 reported lower average content of phenolic acids, as they quantified 2.54 mg kg−1 for polyfloral and 1.88 mg kg−1 for honeydew honey samples. Salonen et al.24 reported the same order of magnitude as in this study, for Nordic honey. Comparing obtained results for individual phenolic compounds with the results of some other authors10,20,24,26 a sufficient number of phenolic compounds with similar values can be noticed (Table S2†).
In general, as it was for sugar content, it could be noticed a higher content of phenolic compounds for polyfloral honey samples. Although, the levels of quantified phenolic compounds are lower than in another foods,31 it is important to considered their sums in honey samples and ability of polyphenols for synergism. In addition, the obtained quantified content for individual phenolic compounds of the present study with the finding results from countries in region, such as honey from Romania,5 Serbia,10 Croatia20 and Slovenia29 suggest that Montenegrin honey contains higher amounts of phenolic compounds.
Antioxidant activity
Descriptive analysis of obtained results of TPC and RSA in honey samples is shown in Table 3. Average value of TPC for honeydew honey is 72.88 mg GAE/100 g and for polyfloral samples is 70.02 mg GAE/100 g. Obtained results are similar to the other reported results for polyfloral samples.10,23 For honeydew honey, there are some studies of higher,5,11,26 and lower reported average values.2,28 Average value of RSA for polyfloral honey is 8.16% (8.80 μmol/100 g) and for honeydew honey samples is 10.01% (10.86 μmol/100 g) (Table 3). Similar variability between samples, but with higher average value (15.83%) was reported for Croatian honeydew honey.32 Larger variability was reported for Romanian honeydew honey samples5 with their higher average value of 23.3%. Similar obtained values of TPC and RSA for polyfloral honey were reported by Ciucure et al.5 and Gašić et al.10Besides the variations within the range of TPC and RSA that are more noticeable for polyfloral samples, there is high similarity between polyfloral and honeydew honey (Fig. S3†). This is contrary to other obtained conclusions stating that the highest antioxidant activity is in honeydew honey, compared to polyfloral honey.2
By determining the Pearson coefficient, a correlation regression analysis was used for comparison of TPC, RSA (spectrophotometric results) and sum of phenolic compounds (chromatographically obtained results) (Table S3†). As a measure of linear correlation between the two variables, high values of Pearson coefficient were noticed for TPC and RSA, 0.907 for honeydew honey and 0.928 for polyfloral honey (Table S3†). Considering it as a satisfactory regression coefficient, it indicates higher total phenolic compounds content and consequently higher antioxidant capacity. It can be assumed that the antioxidant activity is possibly based on the content of phenolic compounds as other suggested.10,23 Differently, there is unsatisfactory Pearson coefficient for sum of phenolic compounds. The assumption for negative correlation (−0.305, −0.012) (Table S3†) can be seen in the numeric obtained values for some samples. Expressed values (high or low) have a significant influence on correlation, especially for a small number of variables. Although one sample should not distort the assessment of correlations, however, when there is a total of eight samples, it is very important. Low correlations (0.061, 0.018, Table S3†) can indicate that TPC and RSA were not changed in the same way as sum of phenolic compounds. It gives rise to independent change of chromatographically determined value in relation to spectrophotometrically.
The results suggested that the values of the individual phenolic compounds in the honey samples were insufficient to have a significant antioxidant effect. However, antioxidant activity can be noticed if the results of the sums of phenolic compounds and results of TPC and RSA values are observed (Table 3). As phenolic compounds show synergism, their biological activity depends on many factors, as well as the ratio of pure components in the mixture, also on other present compounds in honey such as peptides, organic acids, enzymes, Maillard's reaction products, in which combined activities and the interaction can be expose antioxidativity.12,25
Statistical analysis
In order to mark the factors which could be used for differentiation of certain source of bees production, nectar or honeydew, data analysis which includes Mann–Whitney U-test and PCA, was performed on physicochemical parameters, sugar and phenolic compounds content, and TPC and RSA values.Mann–Whitney U-test, used to compare the medians of 49 parameters of honeydew and polyfloral honey, revealed that only electrical conductivity among physicochemical parameters, turanose among sugars and luteolin and quercetin-3-O-galactoside among phenolic compounds, have p values below 0.05. These parameters therefore indicate statistically significant difference between honeydew and polyfloral honey and could be selected as the most discriminating factors. It must be emphasized that only 4 out of 49 parameters determined in analyzed samples is as small number of discriminating factors but also expected number taking into account a fact that honeydew honey and polyfloral honey represent a complex mixture of honeydew and nectar from different botanical species in a relatively small geographical area. Number of parameters which could be used for differentiation between monofloral honey and honeydew honey is much higher due to the domination of nectar of certain, particular plant in monofloral honey.5,15,16,29
For the task of differentiation and classification of honey it is better to combine the several groups of parameters than to observe only one class of compounds, such as sugars, or phenolic compounds, or elements, etc. However, physicochemical parameters mainly gave a good classification results when applied alone, while all other analytical parameters should be combined to evaluate better results.33 In order to evaluate whether physicochemical parameters, on one side, and combining analytical parameters, on the other, could differentiate honeydew honey from polyfloral honey, the data was subjected to pattern recognition analysis.
PCA performed on five physicochemical parameters, water content, electrical conductivity, free acidity, diastase activity and HMF, resulted in three component model which explained 79.64% of the total variance. Mutual projection of factor scores (Fig. S4†) revealed two groups of objects which correspond to honeydew and polyfloral honey. Grouping of honeydew honey was imposed by higher values of electrical conductivity and free acidity; while polyfloral honey was separated according to higher water and HMF content (Fig. S4†).
PCA derived on the initial data matrix, which included all determined parameters, resulted in a model with low percent of variability in the first few principal components. However, observing only those variables showed significant influence on the first PCA model and were additionally matched with the results of Mann–Whitney U-test, new, improved model was obtained. Three-component model explaining 92.62% of variability revealed grouping of samples according to botanical origin (Fig. 2a). The obtained model pointed out that only electrical conductivity could be marked as important factor, which discriminates and characterizes honeydew honey, while turanose, luteolin and quercetin-3-O-galactoside are more abundant in polyfloral honey (Fig. 2b). These results also revealed a higher nutritive value of polyfloral honey compared to honeydew honey, as far as honey from Montenegro is concerned.
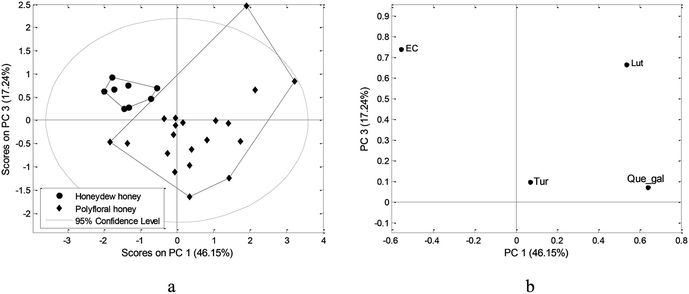 | ||
| Fig. 2 PCA applied on physiochemical parameters, sugar and phenolic compounds content: (a) score plot, (b) loading plot. | ||
Conclusions
This research represents a detail physicochemical analysis of the Montenegrin honey. In the present study, among 28 analyzed honey samples, eight of them were honeydew honey and twenty were polyfloral honey samples, differentiated by electrical conductivity.Based on the obtained results, it can be seen that all investigated honey samples possess considerable nutrition value containing a notable number of phenolic compounds. Generally, there are great similarity between examined honeydew honey and polyfloral honey samples.
Comparing the obtained results of phenolic analysis with the results from countries in the region, such as Serbia, Slovenia, Croatia, Bulgaria, a higher antioxidant potential of honey from Montenegro can be noticed.
It can be concluded that honeydew honey and polyfloral honey from small geographical areas such as northern part of Montenegro, represent a complex mixture of honeydew and nectar from different botanical species, which can cause of similarity of samples.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, through the projects 172017, and III45014. The authors would like to thank to the beekeepers from “The Association of the Beekeeping Organizations of Montenegro” for providing the honey samples. The authors are also grateful to others from Department of Analytical Chemistry of Faculty of Chemistry, University of Belgrade, who helped in the experimental part.References
- Directive 2014/63/EU of the European Parliament and of the Council amending Council Directive 2001/110/EC relating to honey, Official Journal of the European Communities, 2014, L164, https://www.fsai.ie/uploadedFiles/Consol_Dir2001_110.pdf Search PubMed.
- J. Bertoncelj, U. Dobreršek, M. Jamnik and T. Golob, Food Chem., 2007, 105(2), 822–828 CrossRef CAS.
- Z. Can, O. Yildiz, H. Sahin, E. A. Turumtay, S. Silici and S. Kolayli, Food Chem., 2015, 180, 133–141 CrossRef CAS.
- S. K. T. Seraglio, A. C. Valese, H. Daguer, G. Bergamo, M. Z. Azevedo, P. Nehring, L. V. Gonzaga, R. Fett and A. C. O. Costa, Food Res. Int., 2017, 99, 670–678 CrossRef CAS PubMed.
- C. T. Ciucure and E.-I. Geana, Phytochem. Anal., 2019, 30(4), 1–12 CrossRef PubMed.
- C. Pita-Calvo and M. Vázquez, Trends Food Sci. Technol., 2017, 59, 79–87 CrossRef CAS.
- C. Pita-Calvo and M. Vázquez, J. Agric. Food Chem., 2018, 66, 2523–2537 CrossRef CAS PubMed.
- S. Bogdanov, Bee Product Science, International Honey Commision, 2009, http://www.bee-hexagon.net/en/network.htm Search PubMed.
- U. Gašić, M. M. Natić, D. M. Mišić, D. V. Lušić, D. M. Milojković-Opsenica, Ž. Lj. Tešić and D. Lušić, J. Food Compos. Anal., 2015, 44, 128–138 CrossRef.
- U. Gašić, S. Kečkeš, D. Dabić, J. Trifković, D. Milojković-Opsenica, M. Natić and Ž. Tešić, Food Chem., 2014, 145, 599–607 CrossRef PubMed.
- A. Meda, C. E. Lamien, M. Romito, J. Millogo and O. G. Nacoulma, Food Chem., 2005, 91, 571–577 CrossRef CAS.
- L. S. Chua, N. L. A. Rahaman, N. A. Adnan and T. T. E. Tan, J. Anal. Methods Chem., 2013, 2013, 1–8 CrossRef PubMed.
- A. C. Soria, M. González, C. de Lorenzo, I. Martinez-Castro and J. Sanz, J. Sci. Food Agric., 2005, 85, 817–824 CrossRef CAS.
- S. Popek, M. Halagarda and K. Kursa, LWT – Food Sci. Technol., 2017, 77, 482–487 CrossRef CAS.
- I. Escriche, M. Kadar, M. Juan-Borras and E. Domenech, Food Chem., 2014, 142, 135–143 CrossRef CAS PubMed.
- M. Oroian and R. Sorina, Comput. Electron. Agric., 2017, 138, 148–156 CrossRef.
- S. K. T. Seraglio, B. da Silva, G. Bergamo, P. Brugnerotto, L. V. Gonzaga, R. Fett and A. C. O. Costa, Food Res. Int., 2019, 119, 44–66 CrossRef CAS PubMed.
- K. Matović, J. Ćirić, V. Kaljević, N. Nedić, G. Jevtić, N. Vasković and M. Ž. Baltić, Environ. Sci. Pollut. Res., 2018, 25(14), 14148–14157 CrossRef PubMed.
- J. Atanasova, M. Lazarova and L. Yurkova, J. Cent. Eur. Agric., 2016, 17(3), 640–651 CrossRef.
- V. Vasić, U. Gašić, D. Stanković, D. Lušić, D. Vukić-Lušić, D. Milojković-Opsenica, Ž. Tešić and J. Trifković, Food Chem., 2019, 274, 629–641 CrossRef PubMed.
- G. Bergamo, S. K. T. Seraglio, L. V. Gonzaga, R. Fett and A. C. O. Costa, Food Res. Int., 2019, 116, 745–754 CrossRef CAS PubMed.
- I. K. Karabagias, E. Dimitriou, S. Kontakos and M. G. Kontominas, Eur. Food Res. Technol., 2016, 242(8), 1201–1210 CrossRef CAS.
- K. S. do Nascimento, J. A. G. Sattler, L. F. L. Macedo, C. V. S. González, I. L. P. de Melo, E. da S. Araújo, D. Granato, A. Sattler and L. B. de Almeida-Muradian, LWT–Food Sci. Technol., 2018, 91, 85–94 CrossRef.
- A. Salonen, V. Virjamo, P. Tammela, L. Fauch and R. Julkunen-Tiitto, Food Chem., 2017, 237, 214–224 CrossRef CAS PubMed.
- P. Combarros-Fuertes, L. M. Estevinho, L. G. Dias, J. M. Castro, F. A. Tomás-Barberán, M. E. Tornadijo and J. M. Fresno-Baro, J. Agric. Food Chem., 2019, 67, 688–698 CrossRef CAS PubMed.
- R. Halouzka, P. Tarkowski and S. Ćavar-Zeljković, Czech J. Food Sci., 2016, 34(3), 244–253 CrossRef CAS.
- L. Vela, C. de Lorenzo and R. A. Pérez, J. Sci. Food Agric., 2007, 87, 1069–1075 CrossRef CAS.
- R. Socha, L. Juszczak, S. Pietrzyk, D. Gałkowska, T. Fortuna and T. Witczak, Int. J. Food Sci. Technol., 2011, 46, 528–534 CrossRef CAS.
- J. Bertoncelj, T. Polak, U. Kropf, M. Korošec and T. Golob, Food Chem., 2011, 127, 296–302 CrossRef CAS.
- F. A. Tomás-Barberán, I. Martos, F. Ferreres, B. S. Radovic and E. Anklam, J. Sci. Food Agric., 2001, 81, 485–496 CrossRef.
- G. L. Hostetler, R. A. Ralston and S. J. Schwartz, Adv. Nutr., 2017, 8(3), 423–435 CrossRef CAS PubMed.
- D. Broznić, I. Ratkaj, M. Malenica-Staver, S. Kraljević-Pavelić, P. Žurga, D. Bubalo and I. Gobin, Food Technol. Biotechnol., 2018, 56(4), 533–545 Search PubMed.
- H. M. Habib, F. T. Al Meqbali, H. Kamal, U. D. Souka and W. H. Ibrahim, Food Chem., 2014, 153, 35–43 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08783d |
| This journal is © The Royal Society of Chemistry 2020 |