Open Access Article
Open Access ArticleMembrane shell permeability of Rs-198 microcapsules and their ability for growth promoting bioactivity compound releasing
Zhansheng Wu *ab,
Xuan Lib,
Xiaochen Liua,
Jiawei Dongb,
Daidi Fan
*ab,
Xuan Lib,
Xiaochen Liua,
Jiawei Dongb,
Daidi Fan ac,
Xiaolin Xu
ac,
Xiaolin Xu b and
Yanhui He*b
b and
Yanhui He*b
aSchool of Environmental and Chemical Engineering, Xi'an Polytechnic University, Xi'an 710048, P. R. China. E-mail: wuzhans@126.com
bSchool of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832003, P. R. China. E-mail: hyh1083515552@163.com; Fax: +86-993-2057270; Tel: +86-993-2055015
cDepartment of Chemical Engineering, Northwest University, Xi'an 710069, P. R. Chin
First published on 7th January 2020
Abstract
Microencapsulation of bacteria is an alternative technology to enhance viability during processing and application. Different from drug microcapsules, controlled release of the dynamic target bacteria and its metabolites from capsules to the rhizosphere is becoming an important issue for the success of microencapsulated PGPR. This work describes in detail the diffusion permeability coefficient (DPC) of rhizobacteria-loaded microcapsules and their relationship to growth metabolite release and bacterial survival rate. Results showed that the DPC value increased with the decrease of the molecular weight of the model probes, and also the increase of sodium alginate and bentonite concentrations. Importantly, the DPC value was negatively correlated with the survival rate of Rs-198. For storage features, the microencapsulation had no effect on the abilities of IAA production (65 μg mL−1), ammonia nitrogen production (43 mg mL−1), or phosphate dissolved (53 mg L−1) after storing for 90 days. A pot experiment revealed that total nitrogen and phosphorus content of cotton plants in the microcapsules with Rs-198 treatment was increased by 43.48% and 46.51%; soil available phosphorus, ammonium nitrogen, alkali-hydrolysis nitrogen, and nitrate nitrogen contents also increased by 45.43%, 31.48%, 17.13% and 55.69%, respectively due to the synergistic effects of Pseudomonas putida Rs-198 and alginate–bentonite microcapsules. In conclusion, the DPC shows that microcapsules have appropriate permeability to control the bacterial growth metabolism and thus show a beneficial effect on cotton growth. This paper reports the release profile of the bacterial growth metabolism from live rhizobacteria-loaded microcapsules, and will provide valuable guidance on living microcapsule application.
1. Introduction
The growth promoting mechanisms of plant growth-promoting rhizobacteria (PGPR) include direct regulation of phytohormone production, phosphate solubilization, and nitrogen fixation to enhance nutrient acquisition or indirect mechanisms that suppress pathogens by antibiosis, synthesis of lytic enzymes and induced systemic resistance (ISR).1,2 In order to maintain a sufficient number of viable and biologically active PGPR during transportation and storage processes, microencapsulation is an alternative strategy to protect bacteria from adverse external environments.3,4 Many studies have reported that the survival rate of viable cells was higher in alginate microcapsules, as compared to that in free cell bacterial agents under stress conditions.5,6 The permeability of microcapsules is a key index to keep bacteria alive and plays an important role in cell function, nutrient transport, pH maintenance, osmotic adjustment and water penetration.7 Diffusion experiments using different probes showed that the permeability coefficients of the microcapsules decreased with increasing mechanical strength.7 While today's permeability coefficient studies focus on drug release or other sensitive material, fewer studies on the live bacteria loaded microcapsules have been performed. As we all know, the bacteria and its growth metabolites in microcapsules are highly dynamic and are mainly driven by the changes of micro-environment.A better strategy to evaluate the diffusion permeability coefficient (DPC) of bacteria loaded microcapsules is to use growth metabolites likely marker materials to monitor their releasing behaviors. In addition, pH, temperature and salt concentration are important factors in soil that affect the release of cell or secondary metabolites from microcapsules, and thus those factors also should be studied on the influence of DPC.8,9 The diffusional permeabilities of suitable molecule solutes such as VB12 and 4 kDa FITC–dextran across the capsule membranes at pH 4 are lower than those at pH 5.9 Wei et al. reported that the permeability coefficient of VB12 molecules (PVB12) from microcapsule becomes higher with increasing temperature.10 Consequently, how the pH, salt concentration and temperature affect the DPC of live bacteria-loaded microcapsules is very interesting and innovative.
In our previous study, Pseudomonas putida Rs-198 had been encapsulated into alginate (NaAlg)–bentonite (Bent) microcapsules by external gelation technique.11 The near-spherical microcapsules were monodispersed with a mean diameter of 25–100 μm and wrinkled surfaces; and better survival of encapsulated cell was observed, compared to the free cell, especially in pH 4.0 and 10.0. The protective effect of NaAlg–Bent microcapsules on the Rs-198 was mainly through the protective barrier formed by the microcapsule wall for blocking or delaying the entry of substances from the environment.12–15 One of the effective ways for microencapsulated PGPR to work out is to control the release rate of the bacteria from capsules to rhizosphere. These bacteria increase the soil nutrient bioavailability through nitrogen fixation and key nutrients mobilization (phosphorus, potassium and iron), as well as promoting plant growth for the production of indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylate (ACC) deaminase etc. However, less attention has been paid to the effects PGPR microcapsules on its production of IAA (175 Da), ammonia nitrogen (<100 Da), organic acids, or other secondary metabolites. Also, it is not clear how those secondary metabolites in microcapsules transfer and activate to the plant rhizosphere soil. Therefore, this study will focus on determining how environment factors correspond to the diffusion permeability coefficient of microcapsules, which is a indicator of the ability of ions or small molecules to diffuse across the shell, and it is important on bacteria survival rate.8,16
We hypothesized that the metabolites produced by Rs-198 could be released to plant roots from microcapsules. So, the diffusion permeability of five types of microcapsules in different environmental condition was clarified in this study. The correlation between DPC and survival rate of the cells was also analyzed. In addition, growth metabolism of the bacteria in microcapsules was studied via monitoring the contents of the IAA, phosphate solubilization and ammonia in fermentation liquid to confirm that growth metabolites can be released from microcapsules. Pot experiments were carried out to investigate the growth-promoting potential of microencapsulated bio-fertilizer.
2. Materials and methods
2.1 Materials
The raw Bent samples were collected from Xinjiang China Non-metal Xiazijie Bentonite Co., Ltd. The resultant Bent has a composition (%, by mass) of Al2O3 13.06, SiO2 64.62, Na2O 2.66, K2O 2.43, CaO 1.92, MgO 2.38, Fe2O3 4.93, TiO2 0.59, MnO 0.26, and P2O5 0.18, and an ignition loss of 6.20.Nutrient agar medium (NA): 3 g beef extract, 10 g peptone, 5 g NaCl, 18 g agar, 1000 mL H2O, pH 7.0–7.2. Nutrient broth medium (NB): 3 g beef extract, 10 g peptone, 5 g NaCl, (Beijing, China), 1000 mL H2O, pH 7.0–7.2. Pikovskaya's medium: glucose 10 g, Ca3(PO4)2 5 g, yeast extract 0.5 g, (NH4)2SO4 0.5 g, NaCl 0.2 g, KCl 0.2 g, MgSO4·7H2O 0.1 g, MnSO4 1 mL (0.004 g L−1), FeSO4 0.1 mL (0.002 g L−1), H2O 1000 mL, pH 7.48.
2.2 Strain used
The bacteria Rs-198 was isolated from the healthy cotton grown rhizosphere soil in a salinization field of Xinjiang, China, and identified as Pseudomonas putida (GenBank accession no. FJ788425).17 The strain was incubated in fresh nutrient medium and inoculated in a shaker at 30 °C for 24 h to stable stage for further use.2.3 Preparation of microcapsules
The encapsulation of Rs-198 cell were conducted using external gelation technique as described previously.11 10 mL bacteria suspensions (11.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu mL−1) were suspended in 30 mL sodium alginate (NaAlg)–bentonite (Bent) solution. The wall material NaAlg–Bent solution with different proportions (Table 1) containing bacteria suspensions was mixed with 100 mL of liquid paraffin, 1 mL of span 80. Then the mixture was stirred for 25 min with a mechanical stirrer at 1000 rpm (IKA RW20 digital, Germany). The raw bacteria-loaded microcapsules (MB) were hardened by adding CaCl2 solution (2% w/v) with further stirring at 200 rpm for 40 min. The microcapsules were collected by centrifugation at 6576g (≈8000 rpm) and 4 °C for 10 min (TGL 20M, Kaida, Hunan, China).
log cfu mL−1) were suspended in 30 mL sodium alginate (NaAlg)–bentonite (Bent) solution. The wall material NaAlg–Bent solution with different proportions (Table 1) containing bacteria suspensions was mixed with 100 mL of liquid paraffin, 1 mL of span 80. Then the mixture was stirred for 25 min with a mechanical stirrer at 1000 rpm (IKA RW20 digital, Germany). The raw bacteria-loaded microcapsules (MB) were hardened by adding CaCl2 solution (2% w/v) with further stirring at 200 rpm for 40 min. The microcapsules were collected by centrifugation at 6576g (≈8000 rpm) and 4 °C for 10 min (TGL 20M, Kaida, Hunan, China).
| Samples | NaAlg (g g−1) | Bent (g g−1) | Diameter (μm) | Span factor | Encapsulation efficiency (%) | Pore size (nm) |
|---|---|---|---|---|---|---|
| a Different letters on each column are significantly different at p < 0.05. | ||||||
| MBA | 0.5% | 1.0% | 45 ± 4 d | 1.72 ± 0.04 c | 69 ± 2 d | 14.50 ± 0.20 a |
| MBB | 1.0% | 1.0% | 67 ± 4 c | 2.40 ± 0.10 b | 88 ± 2 c | 9.90 ± 0.10 b |
| MBC | 1.5% | 1.0% | 95 ± 3 a | 4.92 ± 0.09 a | 97 ± 2 a | 5.95 ± 0.08 e |
| MBD | 1.5% | 0.5% | 74 ± 6 b | 1.17 ± 0.06 d | 97 ± 2 a | 8.04 ± 0.05 d |
| MBE | 1.5% | 0% | 47 ± 5 d | 0.81 ± 0.03 e | 93 ± 2 b | 8.63 ± 0.04 c |
2.4 Size distribution and span coefficient of microcapsules
Microcapsules (2 mL) were dispersed into 30 mL deionized water and stirred about 10 min before particle size measured. Laser particle size analyzer (Microtrac S3500, USA) was applied to analysis the size distribution of microcapsules. Three replicates were carried out for each formulation.
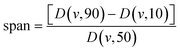 | (1) |
D (v, 90), D (v, 10), and D (v, 50) represent the cumulative volume at 90%, 10%, and 50%, respectively.
2.5 Microcapsule surface mean aperture determination
N2 adsorption–desorption experiments were performed using a specific surface area analyzer (BET, ASAP 2460, Micromeritics, USA) to obtain the average surface pore size of the microcapsules.2.6 Encapsulation efficiency
The encapsulation efficiency was expressed as the strains loaded in the microparticles. The total bacterial cells added in the reaction system denoted as E0 and the number of the unembedded bacteria denoted as Eu could be determined by plate count method. All the experiments were carried out in triplicate. Then the encapsulation efficiency of bacteria could be calculated as follows:
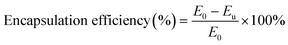 | (2) |
2.7 Diffusion permeation of microcapsules
There is no literature reported about the membrane DPC of secondary metabolites produced by bacteria from microcapsules. There are only some literature shows some solute materials for evaluating the microcapsules DPC.18 These selected markers should be enough to penetrate microcapsules and not interact (adsorption, ionic interaction, etc.) with the microcapsule materials. Glucose, vitamin B12 (VB12) and polyethylene glycols (PEG) have been selected as model solutes for investigating diffusion characteristics of calcium-alginate (Ca-alginate) hydrogel beads and membranes, because of their advantages such as easy-to-gain, easy-to-measure, water-soluble and chemically stable in water.16,18 Bovine serum albumin (BSA, 64.44 kDa), a spherically shaped molecule having an approximately hydrated radius of 35 Å and a pI = 3.9 was also used.19 Based on the consideration above, glucose (180 Da), VB12 (1355 Da), PEG 10000, and BSA (64.44 kDa) were selected as model probes to monitor the DPC of secondary metabolites produced by PGPR inside microcapsules. To load glucose, VB12, PEG into microcapsules, each kind of microcapsules was separately immersed in a 0.1 mg mL−1 glucose solution, 50 μg mL−1 VB12 solution, 10 mg mL−1 PEG buffer solution with 0.15 M NaCl solution at room temperature for 72 h. The buffer solution was refreshed every 24 h. Due to the large molecular weight, BSA was incorporated into wall material before encapsulation process. The microcapsules were harvested and washed quickly 3 times with deionized water. 10 g of the loaded microcapsules were added into 100 mL release medium and incubated in the shaker at 170 rpm. The concentration of markers released in the surrounding medium was measured at regular intervals.2 mL of the test solution and 6 mL of anthrone reagent were placed at boiling water bath for 15 min. Then the glucose concentration was measured using UV-752N Spectrometer (Yuanxi, Shanghai, China) at 620 nm. The VB12 concentration was measured using UV-752N Spectrometer at 361 nm. 0.8 mL 0.05 mol mL−1 I2 solution and 1.2 mL 5% BaCl2 solution were added into 8 mL samples. After 6 min, the PEG was measured using UV-752N Spectrometer at 485 nm. After 2 mL of BSA solution and 10 mL Coomassie brilliant blue G250 were shaken for 2–3 minutes, the BSA concentration was measured using UV-752N Spectrometer at 595 nm.
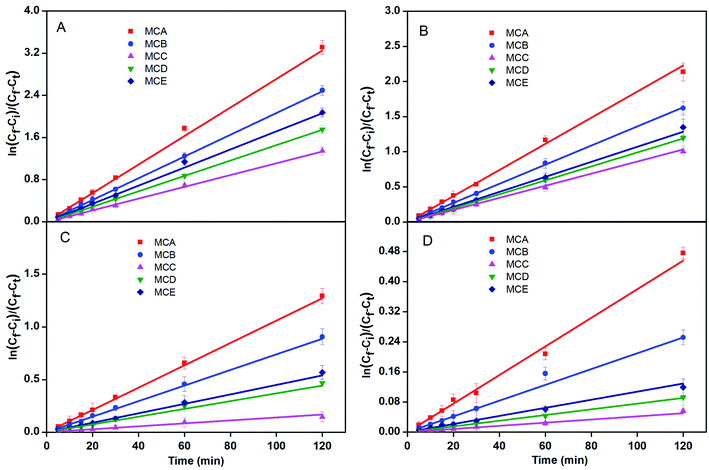
According to Fick's first law, the DPC of the microcapsule for glucose, VB12, PEG and BSA was calculated with eqn (3):
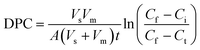 | (3) |
Since ln{(Cf − Ci)/(Cf − Ct)} has a linear relationship with time t, the formula could be simplified as:
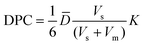 | (4) |
![[D with combining macron]](https://www.rsc.org/images/entities/i_char_0044_0304.gif) was the average particle size of the microcapsules, K is the slope of the straight line obtained by plotting ln{(Cf − Ci)/(Cf − Ct)} versus t.
was the average particle size of the microcapsules, K is the slope of the straight line obtained by plotting ln{(Cf − Ci)/(Cf − Ct)} versus t.
2.8 Survival rate testing
For survival rate: 1 g of the microcapsules and 1 mL free bacteria were incubated in 9 mL solution (stored at different temperature, pH, salt concentration conditions). The initial bacteria density (N0) in microcapsules was about 3.68 × 109 cfu g−1 and free bacteria were about 2.29 × 109 cfu mL−1. The NaAlg–Bent microcapsules were broken by a chemical method after 90 days and the number of the cells was recorded as Nt. The survival rate was calculated by Nt/N0.For cell growth and leakage: cell growth of bacteria-loaded microcapsules during culture process: 1 g microcapsules (MBC, 9.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1) were inoculated with 9 mL of NB medium and cultured in a shaking incubator at 30 °C, 170 rpm for 48 h. The cultured NaAlg–Bent microcapsules at the appointed time were broken by a chemical method and the number of the cells in microcapsules were collected and recorded as internal.20 The bacteria leaked into the medium also collected at same appointed time and recorded as leakage. All cell experiments were carried out in triplicate samples.
log cfu g−1) were inoculated with 9 mL of NB medium and cultured in a shaking incubator at 30 °C, 170 rpm for 48 h. The cultured NaAlg–Bent microcapsules at the appointed time were broken by a chemical method and the number of the cells in microcapsules were collected and recorded as internal.20 The bacteria leaked into the medium also collected at same appointed time and recorded as leakage. All cell experiments were carried out in triplicate samples.
2.9 Growth promoting property
Indole-3-acetic acid (IAA) production by Rs-198: 300 μL microcapsules samples were inoculated in 30 mL NB medium in 50 mL beaker flask for 36 h, 30 °C at 170 rpm. The fermentation was centrifuged at 6576 × g for 10 min and the supernatant was collected for further use. IAA concentration was quantified using Salkowski reagent colorimetric method at 530 nm in UV-752N ultraviolet spectrophotometer. Each experiment was conducted in triplicate. Ammonia nitrogen: the microcapsules or the free Rs-198 were cultivated at 30 mL peptone water (10 g L−1 peptone, 5.0 g L−1 NaCl, H2O 1000 mL, pH 7.0) for 36 h at 30 °C and 170 rpm. For ammonia production quantitative analysis, the supernatant was collected via the fermentation centrifuged at 6576 × g for 10 min. Then the ammonia content was tested according to the Nessler's reagent spectrophotometry method at 420 nm.21 Each experiment was conducted in triplicate. Phosphate-solubilizing capacity: 300 μL samples were taken at different time intervals in 0.9% NaCl solution. Then samples cultures were grown in 30 mL Pikovskaya's medium at 50 mL beaker flask for 36 h, 30 °C at 170 rpm. The supernatant was harvested by centrifugation at 6576 × g (10 min) for further experiments. The capacity of phosphate-solubilizing was measured by Mo–Sb colorimetry at 700 nm using a UV-752N ultraviolet spectrophotometer. All experiments were carried out in triplicate samples. Number of live bacteria: 1 g of the wet microcapsules (MBC) and 1 mL of the free bacteria were incubated in 9 mL solution (stored at different 25 °C, pH 7, 0.9% salt concentration conditions for 90 days). The viable bacteria released into the solution as well as free bacteria were determined by plate count method at various time intervals.2.10 Pot experiment
Plump cotton seeds of uniform size were disinfected by 75% alcohol for 5 min twice, and then washed with sterile water three times. The seeds were put on filter paper for drying surface water. 10 cotton seeds were sown into the pot containing 100 g soil. The pot experiment treatments include blank group (CK), free bacterial agent (0.5 mL 109 cfu mL−1) (FB), NaAlg alone (0.5 g) (NaAlg), and Bent alone (0.5 g) (Bent), the microcapsules without bacteria (0.5 g) (MW), microencapsulated bacterial agent (0.5 g). Five replicates were treated for each treatment. Plants were watered as needed with autoclaved water. The soil used in this study was collected from Agricultural Test Site of Shihezi University (44°17′N, 85°49′E), located in Xinjiang, China and the soil is classified as slightly-saline, desert grey soil. The basic soil properties of this soil were as follow: pH 8.16, 0.54 g kg−1 of total-N, 0.93 g kg−1 of total-P, 15.20 g kg−1 of organic matter, 28.70 mg kg−1 of available phosphorus, 57.90 mg kg−1 of nitrate nitrogen, 21.83 mg kg−1 of ammonium nitrogen and 86.60 mg kg−1 of alkali-hydrolysis nitrogen. The total nitrogen and total phosphorus contents of cotton plants in soil pot were measured according to the book of Soil Agro-Chemistry Analysis.22,23 The available phosphorus, NO3−–N, NH4+–N and nitrogen of soil were measured according to the book.2.11 Statistical analysis
The results are reported throughout as mean ± standard deviation and showed as figure created in origin 8.5 Statistical software (Origin Lab, Wellesley, MA, USA). Statistical analysis of the data was conducted using analysis of variance (ANOVA), and Turkey's multiple comparison tests in origin 8.5. Values p < 0.05 was considered statistically significant.3. Results and discussion
3.1 Mean diameter, span and encapsulation efficiency of microcapsules
The average diameter, span factor, encapsulation efficiency and average pore size of microcapsules MBA–MBE were displayed in Table 1. The average diameter and span factor of microcapsules were found to increase from 44.85 μm to 94.74 μm, with the increase of NaAlg and Bent concentration. Because NaAlg and Bent increased the viscosity of the solution, reduce the emulsification of droplets, resulting in the increasing of particle size and span coefficient.12,24 Park et al. also found that the mean size of the microcapsules was related to the viscosity and reunification of core materials.25 Those results also supported by Zhao who reported that the mean diameters of microcapsules increased from 268.2 to 404.6 μm when alginate concentration increased from 2 to 4% (w/w).26The encapsulation efficiency varied from 69.34% to 97.46% with the NaAlg concentration increasing from 0.5% to 1.5%. The cross-linking was strengthened by more NaAlg in wall material reacting with CaCl2 and excellent film formation made encapsulation efficiency increased.15 However, there is no significant difference among MBC, MBD and MBE. The pore size is negatively correlated with, respectively, the concentration of NaAlg (14.49 mm to 5.95 mm) and Bent (8.63 mm to 5.95 mm) under the premise of controlling single variable. The concentration ratio of NaAlg and Bent has an effect on the surface structure of the wall material, and may also affect the release of internal bacteria.
3.2 Diffusion permeability of microcapsule membranes
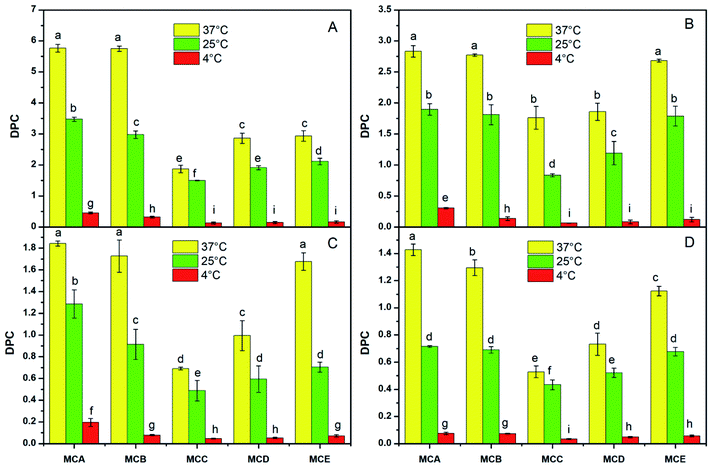
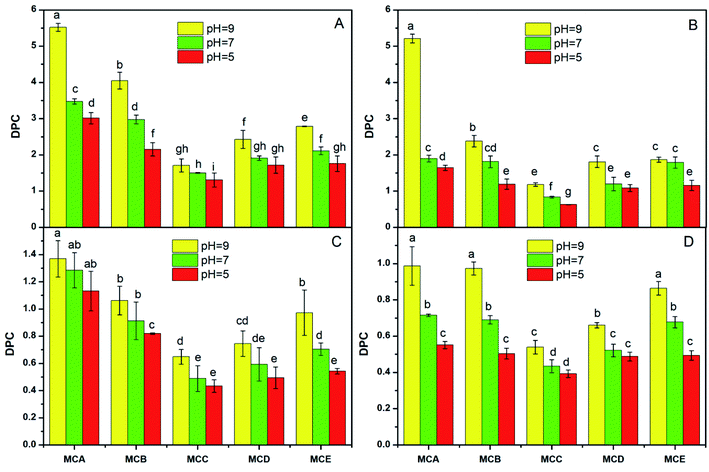
The appropriate permeability coefficient of microcapsules could protect the cells from harsh environment and also maintain cell viability by allowing the exchange of nutrients, oxygen, and waste products between the intra- and extra-microcapsule environments.27 The environmental conditions like pH, temperature, salt concentration had influence on drug microcapsule DPC have been reported in previous study.28 The DPC values of different solute molecule in all kinds of microcapsules significantly declined with the temperature range from 37 °C, 25 °C to 4 °C (Fig. 1). The reasons assigned to higher temperature accelerated molecular motion and the rate of solute molecules entering or leaving of microcapsules, so the DPC value increased with the temperature increased. Wei also reported that the diffusion permeability coefficient (VB12) value of CS-M-T microcapsules increases with increasing of temperature from 25 °C to 49 °C.10The DPC values was lowest when pH was 5.0, whereas highest when pH was 9.0 in the same solute molecule and microcapsules (Fig. 2). This is because the microcapsules likely tend to be shrunken under acidic conditions and swelling at the neutral or even alkaline environment. The results of Zhao also showed that the DPC of microcapsules was much higher than that of bile salt solution (pH 6.8) compared with in simulated gastric fluid (pH 2.0).7 He who used VB12 as tested probes also reported that DPC value increased with the increase of pH.9 In addition, calcium alginate slightly dissolved under alkaline conditions, which also accelerated the release of substances from the microcapsules.
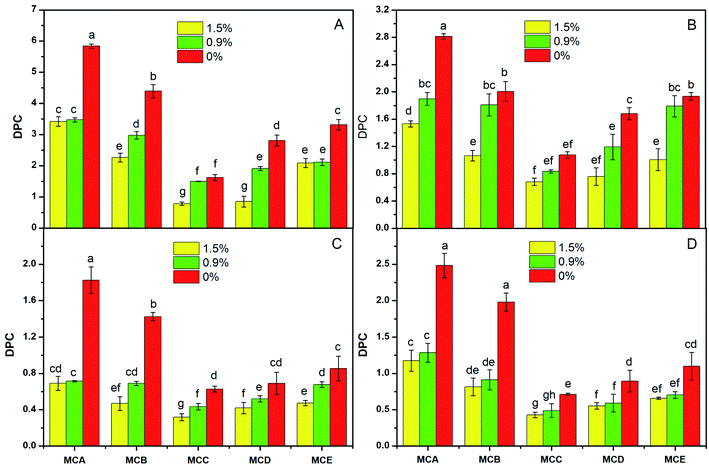
The DPC value decreased with the salt concentration increasing in the same conditions of microcapsules and solute molecules (Fig. 3). The reason may be that the concentration at the 1.5% salt solution resulted an increased external osmotic pressure of the microcapsules, which hindered solute molecules release from the microcapsules; on the contrary, when the microcapsules were in water, the microcapsules internal osmotic pressure was greater than that external, which was favorable for the substances release from microcapsules.
The DPC value change trend was DPCA > DPCB > DPCE > DPCD > DPCC varied with the pH, temperatures, and salt concentration, which were in the same storage conditions and solute molecule (Fig. 1–3). The reason could be contributed that shell formation performance was enhanced by adding NaAlg. Our previous study has shown that the diameter of microcapsules increased with the increasing of NaAlg and Bent concentration, and the MBC showed the biggest diameter (Table 1). This phenomenon shows that large particle size will lead to smaller DPC. Another reason for the DPC value change was due to the pores size of the microcapsules. As is well know, the reduction in the number of pores via adding Bent and NaAlg will cause the decrease of path that substance enter and out of the microcapsules. In the study of Puguan also showed that the diffusion coefficient of VB12 slightly decreased with the increase of the sodium alginate concentration.18 Bentonite could be one of the most possible reasons to this increase, for that bentonite is broadly used for its well water swelling, fine absorption and cation exchange, and it is very conducive to nutrient exchange inside and outside the membrane. With the same environmental conditions and microcapsules, the DPC value decreased with the increasing of the molecular weight of the four solute molecules glucose, VB12, PEG and BSA. Permeability of the APSi microcapsule membranes reported by He also showed that the size match between the solute molecules size and the pore size of APSi capsule membranes had effect on DPC values during the transmembrane diffusion.9 In addition, low molecules weight of the soluble markers has been reported to have higher DPC value.7
The ln{(Cf − Ci)/(Cf − Ct)} were linearly correlated with time t. Obviously, the slope of the straight line was affected by microcapsule type and solute molecular weight. The increase of solute molecular weight showed lower ln{(Cf − Ci)/(Cf − Ct)}. Similar results had also appeared in the study of He, who found that the slopes of the straight lines of ln{(Cf − Ci)/(Cf − Ct)} versus t for methylene blue molecules were higher than that for VB12 molecules, PEG molecules and BSA molecules.9 Besides, the effect of wall material concentration on diffusion permeability of the microcapsule film was MBC > MBD > MBE > MBB > MBA, associated to the pore size of the microcapsule network which related with the alginate and Bent content in wall material (Fig. 4).
 | ||
| Fig. 4 Plot of ln[(Cf − Ci)/(Cf − Ct)] versus t of the glucose (A), VB12 (B), PEG 10000 (C), and BSA (D) molecules across the trans-membrane of five microcapsules at 0.9%, 25 °C and pH 7.0. | ||
3.3 Survival rate of Rs-198 in microcapsules
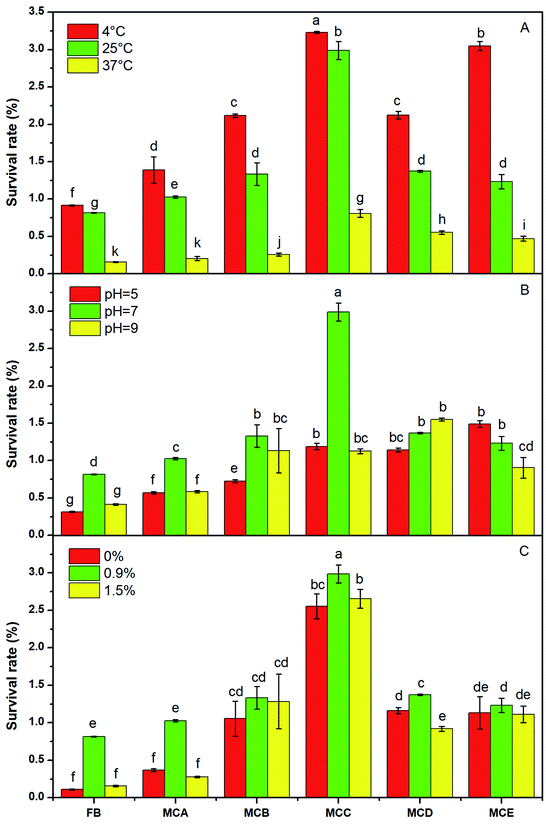
The highest viability of the five microcapsules was at 4 °C, the lowest survival rate was at 37 °C at 90 day (Fig. 5A). This may be because that high storage temperature showed higher bacteria activity and consumed more energy, if bacteria lose their energy, they can't keep normal metabolism and gradually die.29 Other author also reported that the decrease in temperature leads to increase in bacteria stability.30 The viability of the bacteria at in microcapsules always higher than free cell, and pH 7.0 was always higher than that at pH 5.0 and pH 9.0 (Fig. 5B). The external pH affected the activity of cell membrane and extracellular hydrolase, but the membrane surrounding alginate beads reduces transition of acids and alkaline from the solution into the beads, therefore limits their adverse effect on the encapsulated bacteria.31 In the other hands, alginate has a low stability in acidic conditions, it shows less protection on cell at pH 5.0 conditions.The survival rate of MBA was 0.36%, 1.03% and 0.28%, respectively, under salt concentration of 0%, 0.9% and 1.5% at 90 day (Fig. 5C). The highest survival rate at 0.9% salt solution was because the 0.9% salt solution provided a proper osmotic pressure to protect the bacteria. In addition, NaCl acted as a chelator to release Ca2+ from the microcapsules, which led to swelling and dissolution of calcium alginate in microcapsules, thereby facilitating the release of cells from the microcapsules.32 Therefore the bacteria remained in the microcapsules were less for more bacteria released from the dissolution hole of calcium alginate microcapsules at 1.5% salt solution condition.
The survival rate of the bacteria in the MBA, MBB and MBC increased gradually with the increase of NaAlg concentration, for a more densely cross-linked gel structure will be formed, and a better protection of bacterial cells will be achieved.12,15
3.4 The correlation of microcapsules DPC and bacterial survival rate
The survival rate of the bacteria was negatively correlated with the DPC value, this might be assigned to the good permeability of the microcapsule membrane, which is conducive to the release of bacteria (Fig. 6). On one hand, the substances from the external environment could easily enter the interior of the microcapsules, thereby affecting the survival rate of Rs-198 in the microcapsules. On another hand, higher permeability will also lead to more bacteria release to outside of microcapsule and lose of protective shell. It is also proved that microencapsulation in semi-permeable membranes protects cells against immune destruction.33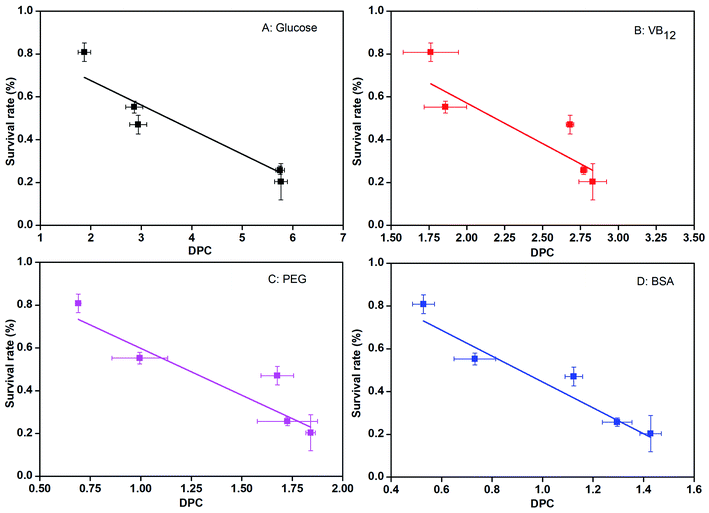 | ||
| Fig. 6 The correlation analysis of DPC of glucose, VB12, PEG 10000, and BSA molecules in microcapsules to survival rate of bacteria in microcapsules. | ||
3.5 Cell density of microcapsulation Rs-198 during cell culture process
The microcapsules (MBC) with lowest permeability were used for bacteria leakage test. The number of bacteria in microcapsules and the leakage from microcapsules during the culture for 48 h were presented in Fig. 4. In the first 18 h, the number of bacteria in NaAlg–Bent microcapsules appeared to have no significant changes, which held about 8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1. The maximum cell number in microcapsules was about 9.18
log cfu g−1. The maximum cell number in microcapsules was about 9.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1 at 30 h. After slight growth, the encapsulated cells were declined until kept at 8.10
log cfu g−1 at 30 h. After slight growth, the encapsulated cells were declined until kept at 8.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1 constantly. The Rs-198 leakage had a long period exponential phase from 0 to 10.17
log cfu g−1 constantly. The Rs-198 leakage had a long period exponential phase from 0 to 10.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1 in 24 h. The leaked Rs-198 kept about 10.20
log cfu g−1 in 24 h. The leaked Rs-198 kept about 10.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cfu g−1 for 12 h (at stable phase) and then went into death phase. Several significant studies have shown that reduce of the permeability of microcapsules wall, resulting in a strong effect for inhibition of drug leakage.34 The microcapsule membrane formed by sodium alginate and calcium ions could provide stable microenvironment and had beneficial effect on the growth and leakage of bacteria.20 Adding bentonites into sodium alginate maybe lead to the inner space of microcapsule shrink and limit the growth and active of bacteria in microcapsules. But the release of Rs-198 into NA medium was due to the hole/pore of the surface and the swelling of the microcapsules. The addition of alginate–chitosan in the wall complex improved mechanical and chemical stability of microcapsules wall, significantly reduced permeability of microcapsules wall,35 resulting less leakage of the entrapped cells into the medium (Fig. 7).
log cfu g−1 for 12 h (at stable phase) and then went into death phase. Several significant studies have shown that reduce of the permeability of microcapsules wall, resulting in a strong effect for inhibition of drug leakage.34 The microcapsule membrane formed by sodium alginate and calcium ions could provide stable microenvironment and had beneficial effect on the growth and leakage of bacteria.20 Adding bentonites into sodium alginate maybe lead to the inner space of microcapsule shrink and limit the growth and active of bacteria in microcapsules. But the release of Rs-198 into NA medium was due to the hole/pore of the surface and the swelling of the microcapsules. The addition of alginate–chitosan in the wall complex improved mechanical and chemical stability of microcapsules wall, significantly reduced permeability of microcapsules wall,35 resulting less leakage of the entrapped cells into the medium (Fig. 7).
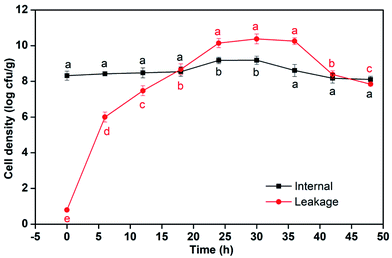 | ||
| Fig. 7 Cell densities in MB microcapsules and leakage bacteria during the culture for 48 h (different letters on each column are significantly different at p < 0.05.). | ||
3.6 Growth and metabolism of bacteria in microcapsules
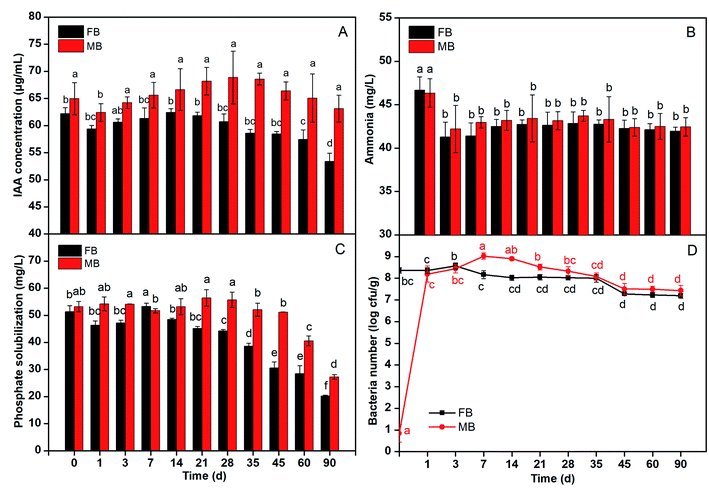
The IAA production of Rs-198 in microcapsules maintained around 65 μg mL−1 in 90 days (Fig. 8A) and the slight fluctuation was resulted from the number of bacteria in samples see Fig. 8D. The viable bacteria kept high number through the storage period. Our previous study had explained the reason for microcapsules provide low-stress microenvironment to cells.12–15 The IAA production of free bacteria at 59 μg mL−1 stored 3 months, and there was similar fluctuation. This might be due to the shell of microcapsules protected the bacteria (Rs-198). We evaluated the ability of the Rs-198 produce ammonia released from microcapsules to under saline storage for 90 days (Fig. 8B). The results have showed that the ability of Rs-198 to produce ammonia, either in microcapsules or in free bacteria, remained essentially same after storage. The phosphate solubilization ability of the free and encapsulated cells in certain time was depicted in Fig. 8C. The phosphate solubilization of cells in microcapsules was slightly varied. It is around 53 mg L−1 in most case, except 60 day (40.6 mg L−1) and 90 day (27.3 mg L−1). The phosphate solubilization ability of free cells held 45 mg L−1 in first 28 days, but gradually decreased after 45 days (30.5 mg L−1), 60 day (28.4 mg L−1) and 90 day (20.2 mg L−1). The microcapsules as a protective shell protected bacteria from unfavorable environment in the first 60 day.The Rs-198 promotes cotton growth and development by a mechanism of IAA production, ammonia production and phosphate solubilization etc. Microencapsulation, as a protective mechanism, did not show bad effect on growth and metabolism of the bacteria. In addition, compared to the ability of IAA and ammonia, the storage day (inoculated amount) had greater effect on the phosphate solubilization ability (Fig. 8). Wang et al. similarly indicated that bacteria can still conserve bacterial ureolytic activity after being immobilized into silica gel and polyurethane foam.36 What's more, result showed that the metabolite produced by bacteria can freely enter and exit the capsule.
3.7 Plant fresh and dry weight
A noteworthy increase in root length, plant height, fresh weight, and dry weight of cotton plants were observed in MB (microencapsulated bacterial agent) treatment (Table 2). Compared with their corresponding control plants, MB treatment significantly increased root length, plant height, fresh weight, and dry weight by 19.48%, 23.47%, 65.36%, and 99.08%, respectively. FB (free bacterial agent) treatment increased fresh and dry weight by 37.86% and 67.88% compared to control plants. What's more, MB treatment can increase fresh weight, by 28.30% compared to FB treatment. These results indicate that a synergetic effect was formed by Rs-198 and NaAlg, Bent.14 MW also significantly increased plant fresh and dry weight by 28.88% and 63.30% to control. This may be because that MW takes advantages of NaAlg and bent for NaAlg as a biodegradable material can be used as an energy and carbon source for other beneficial microorganisms and Bent significantly increased soil moisture, soil water storage, saturated hydraulic conductivity water use efficiency in semi-arid region for its absorption capacity.37 It had been reported application of Ca-alginate-entrapped PGPR in a soil-plant system can be an efficient technique for plant establishment and growth in nutrient deficient soils.38,39| Treatments | Root length (cm) | Plant height (cm) | Fresh weight (g) | Dry weight (g) |
|---|---|---|---|---|
| a Different letters on each column are significantly different at p < 0.05. | ||||
| CK | 6.0 ± 0.5 b | 12 ± 1 b | 0.90 ± 0.10 d | 0.11 ± 0.01 c |
| FB | 6.8 ± 0.4 ab | 13 ± 1 b | 1.20 ± 0.09 b | 0.18 ± 0.03 ab |
| MB | 7.1 ± 0.6 a | 15 ± 1 a | 1.44 ± 0.05 a | 0.22 ± 0.04 a |
| MW | 7.0 ± 0.5 ab | 14 ± 2 ab | 1.12 ± 0.05 bc | 0.18 ± 0.06 ab |
| NaAlg | 6.6 ± 0.6 ab | 13 ± 1 b | 0.90 ± 0.06 cd | 0.15 ± 0.02 bc |
| Bent | 6.1 ± 0.4 b | 13 ± 1 ab | 1.04 ± 0.09 c | 0.15 ± 0.03 b |
3.8 Cotton plant and soil N, P content
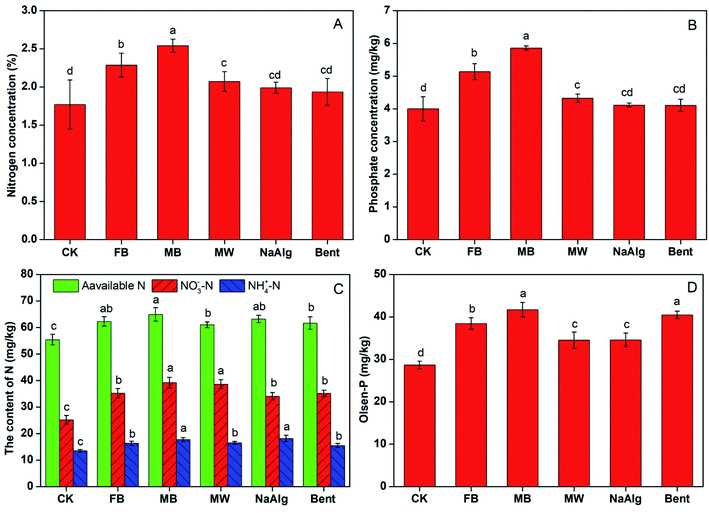
Total N, P concentration of cotton plants in MB treatment were significantly higher compared with FB and MW treatment (Fig. 9A and B). MB treatment showed highest total nitrogen and phosphorus contents in cotton plants, which increased by 43.48% N and 46.51% P compared with CK; followed by FB treatment, which increased by 29.14% N, and 28.45% P. Various microbial inoculants are already reported to promote soil microbial development, nutrient availability and plant growth through processes such as nitrogen fixation, nutrient mobilization, plant growth regulator syntheses in recent years.40,41 These finding may be due to the increased N2 fixation and phosphorus solubilization ability of Rs-198. What's more, more Rs-198 survived in the microcapsules and will cause more available N and P in rhizosphere soil, so MB showed highest total N, P contents. In the research Yadav reported that the inoculation of composite treatments combination of PGPB strains increased the N, P, and K contents in grain and straw of rice at different levels P application.42 These results confirmed that the presence of immobilized beneficial bacteria using microcapsules in the soil substantially enhancing its survival ratio.43MB treatment significantly increased the soil alkali-hydrolysis nitrogen NO3−–N, NH4+–N and Olsen-P content by 17.13%, 55.69%, 31.48% and 45.43%, respectively compared to that control (Fig. 9C and D). What's more, the NO3−–N, NH4+–N and Olsen-P contents in MB treatment were significantly higher than that FB, and MW treatment. Several studies have outlined the direct or indirect role of PGPB in elevations of oil nutrients to promote plant better uptake nutrient. Our previous work had proved that Rs-198 increase the soil P content by increase the phosphate solubilizing bacteria abundance in the pepper rhizosphere soil.44 These results illustrate the additional protection that the microcapsules provide to the bacteria.24 It is important to note that significantly difference in soil alkali-hydrolysis nitrogen NO3−–N, NH4+–N and Olsen-P contents between free bacteria and control, alkali-hydrolysis nitrogen NH4+–N and Olsen-P contents between MB and MW, especially for MB increased by 22.86% in soil Olsen-P than MW. For Rs-198 had been shown to have great growth promoting effect with its phosphate solubilizing property, and nitrogen fixation performance.17 On the other hand, inoculation of Rs-198 microcapsules may associated with microbial diversity changes was also concomitant with soil N, P range.45 Song et al. has proved the synergistic effects vermicompost and PGPR on both soils and crops.46 Another reason for MB increase soil N, P content may be because those microcapsules protect bacteria from harsh environment and increase bacteria population, which can soluble more P and fix more N in the soil.
4. Conclusions
This work describes the diffusion permeability of rhizobacteria-loaded microcapsules and its relationship to growth metabolites release as well as bacteria survival rate in detail. The DPC of microcapsules were negatively related to molecular weight of probe molecules, while positively related to temperature, pH, salt concentration and average pore size of microcapsules. The most important thing is the survival rate of the Rs-198 was negatively correlated with the DPC of the microcapsule membranes. Besides, microencapsulate did not affect IAA, ammonia production and phosphate solubilization ability of microencapsulated Rs-198. Moreover, the study also proved that microencapsulated Rs-198 promoted plant growth on biomass and total N, P contents by improving soil nitrogen, NO3−–N, NH4+–N and Olsen-P content. The results in this study provide valuable guidance for the easy fabrication and widely application of live PGPRs microcapsules formulation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Meg Parmer, Fort Collins, CO, USA, for reviewing and language editing. This work was financially supported by the (National Natural Science Foundation of China) under grant (number 21566035, U1803332); ("Double First Class" Science and Technology Project of Shihezi University) under grant (number SHYL-ZD201805); (China Scholarship Council) under grant number (No. 201709505007).References
- B. R. Glick, Bacteria with ACC deaminase can promote plant growth and help to feed the world, Microbiol. Res., 2014, 169, 30–39 CrossRef CAS PubMed.
- M. Kaushal and S. P. Wani, Rhizobacterial-plant interactions: strategies ensuring plant growth promotion under drought and salinity stress, Agric., Ecosyst. Environ., 2016, 231, 68–78 CrossRef CAS.
- M. Schoebitz, M. D. López and A. Roldán, Bioencapsulation of microbial inoculants for better soil–plant fertilization, Agron. Sustainable Dev., 2013, 33, 751–765 CrossRef CAS.
- J. B. Divya and K. M. Nampoothiri, Encapsulated Lactococcus lactis with enhanced gastrointestinal survival for the development of folate enriched functional foods, Bioresour. Technol., 2015, 188, 226–230 CrossRef CAS PubMed.
- B. Haghshenas, N. Abdullah, Y. Nami, D. Radiah, R. Rosli and A. Yari Khosroushahi, Microencapsulation of probiotic bacteria Lactobacillus plantarum 15 HN using alginate-psyllium-fenugreek polymeric blends, J. Appl. Microbiol., 2015, 118, 1048–1057 CrossRef CAS PubMed.
- R. M. Kent and S. B. Doherty, Probiotic bacteria in infant formula and follow-up formula: Microencapsulation using milk and pea proteins to improve microbiological quality, Food Res. Int., 2014, 64, 567–576 CrossRef CAS PubMed.
- M. Zhao, F. Qu, Z. Wu, K. Nishinari, G. O. Phillips and Y. Fang, Protection mechanism of alginate microcapsules with different mechanical strength for Lactobacillus plantarum ST-III, Food Hydrocolloids, 2017, 66, 396–402 CrossRef CAS.
- Y.-C. Chen, R. Xie and L.-Y. Chu, Stimuli-responsive gating membranes responding to temperature, pH, salt concentration and anion species, J. Membr. Sci., 2013, 442, 206–215 CrossRef CAS.
- F. He, L. Mei, X.-J. Ju, R. Xie, W. Wang, Z. Liu, F. Wu and L.-Y. Chu, pH-responsive controlled release characteristics of solutes with different molecular weights diffusing across membranes of Ca-alginate/protamine/silica hybrid capsules, J. Membr. Sci., 2015, 474, 233–243 CrossRef CAS.
- J. Wei, X.-J. Ju, X.-Y. Zou, R. Xie, W. Wang, Y.-M. Liu and L.-Y. Chu, Multi-Stimuli-Responsive microcapsules for adjustable controlled-release, Adv. Funct. Mater., 2014, 24, 3312–3323 CrossRef CAS.
- X. Li, Z. Wu, Y. He, B.-C. Ye and J. Wang, Preparation and characterization of monodisperse microcapsules with alginate and bentonite via external gelation technique encapsulating Pseudomonas putida Rs-198, J. Biomater. Sci., Polym. Ed., 2017, 28, 1556–1571 CrossRef CAS PubMed.
- Z. S. Wu, Y. H. He, L. Y. Chen, Y. J. Han and C. Li, Characterization of Raoultella planticola Rs-2 microcapsule prepared with a blend of alginate and starch and its release behavior, Carbohydr. Polym., 2014, 110, 259–267 CrossRef CAS PubMed.
- L. Tu, Y. He, C. Shan and Z. Wu, Preparation of microencapsulated Bacillus subtilis SL-13 seed coating agents and their effects on the growth of cotton seedlings, BioMed Res. Int., 2016, 3251357 Search PubMed.
- Y. H. He, Z. S. Wu, B. C. Ye, J. Wang, X. Y. Guan and J. H. Zhang, Viability evaluation of alginate-encapsulated Pseudomonas putida Rs-198 under simulated salt-stress conditions and its effect on cotton growth, Eur. J. Soil Biol., 2016, 75, 135–141 CrossRef.
- Y. H. He, Z. S. Wu, L. Tu, Y. J. Han, G. L. Zhang and C. Li, Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate, Appl. Clay Sci., 2015, 109, 68–75 CrossRef.
- Z. Liu, X.-J. Ju, Y.-H. Huang, R. Xie, W. Wang, K.-R. Lee and L.-Y. Chu, Diffusional permeability characteristics of positively K+-responsive membranes caused by spontaneously changing membrane pore size and surface wettability, J. Membr. Sci., 2016, 497, 328–338 CrossRef CAS.
- L. X. Yao, Z. S. Wu, Y. Y. Zheng, I. Kaleem and C. Li, Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton, Eur. J. Soil Biol., 2010, 46, 49–54 CrossRef CAS.
- J. M. C. Puguan, X. Yu and H. Kim, Diffusion characteristics of different molecular weight solutes in Ca–alginate gel beads, Colloids Surf., A, 2015, 469, 158–165 CrossRef CAS.
- K. R. Smith and R. T. Borchardt, Permeability and mechanism of albumin, cationized albumin, and glycosylated albumin transcellular transport across monolayers of cultured bovine brain capillary endothelial cells, Pharm. Res., 1989, 6, 466–473 CrossRef CAS PubMed.
- H. Song, W. Yu, M. Gao, X. Liu and X. Ma, Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process, Carbohydr. Polym., 2013, 96, 181–189 CrossRef CAS PubMed.
- X. Li, X. Geng, R. Xie, L. Fu, J. Jiang, L. Gao and J. Sun, The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum, Biotechnol. Biofuels, 2016, 9, 190 CrossRef PubMed.
- R. C. Dalal and R. J. Henry, Simultaneous determination of moisture, organic carbon, and total nitrogen by near infrared reflectance spectrophotometry 1, Soil Sci. Soc. Am. J., 1986, 50, 120–123 CrossRef CAS.
- S. Bao, H. Qin and J. Lao, Soil Agro-chemistrical Analysis, China Agriculture Press, 1988 Search PubMed.
- Y. Bashan, L. E. de-Bashan, S. Prabhu and J.-P. Hernandez, Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013), Plant Soil, 2014, 378, 1–33 CrossRef CAS.
- S.-J. Park, Y.-S. Shin and J.-R. Lee, Preparation and characterization of microcapsules containing lemon oil, J. Colloid Interface Sci., 2001, 241, 502–508 CrossRef CAS.
- M. Zhao, F. Qu, S. Cai, Y. Fang, K. Nishinari, G. O. Phillips and F. Jiang, Microencapsulation of Lactobacillus acidophilus CGMCC1. 2686: Correlation between bacteria survivability and physical properties of microcapsules, Food Biophys., 2015, 10, 292–299 CrossRef.
- H. Wen, T. Gao, Z. Fu, X. Liu, J. xu, Y. He, N. Xu, P. Jiao, A. Fan, S. Huang and W. Xue, Enhancement of membrane stability on magnetic responsive hydrogel microcapsules for potential on-demand cell separation, Carbohydr. Polym., 2017, 157, 1451–1460 CrossRef CAS PubMed.
- J. G. Werner, B. T. Deveney, S. Nawar and D. A. Weitz, Dynamic microcapsules with rapid and reversible permeability switching, Adv. Funct. Mater., 2018, 28, 1803385 CrossRef.
- D. Dianawati, S. F. Lim, Y. B. H. Ooi and N. P. Shah, Effect of type of protein-based microcapsules and storage at various ambient temperatures on the survival and heat tolerance of spray dried Lactobacillus acidophilus, J. Food Sci., 2017, 82, 2134–2141 CrossRef CAS PubMed.
- M. T. Sánchez, M. A. Ruiz, A. Lasserrot, M. Hormigo and M. E. Morales, An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study, Food Hydrocolloids, 2017, 69, 67–75 CrossRef.
- H. Gandomi, S. Abbaszadeh, A. Misaghi, S. Bokaie and N. Noori, Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions, LWT--Food Sci. Technol., 2016, 69, 365–371 CrossRef CAS.
- C. H. Liu, J. Y. Wu and J. S. Chang, Diffusion characteristics and controlled release of bacterial fertilizers from modified calcium alginate capsules, Bioresour. Technol., 2008, 99, 1904–1910 CrossRef CAS PubMed.
- J. Dusseault, F. A. Leblond, R. Robitaille, G. Jourdan, J. Tessier, M. Ménard, N. Henley and J.-P. Hallé, Microencapsulation of living cells in semi-permeable membranes with covalently cross-linked layers, Biomaterials, 2005, 26, 1515–1522 CrossRef CAS PubMed.
- J. Shao, M. Xuan, T. Si, L. Dai and Q. He, Biointerfacing polymeric microcapsules for in vivo near-infrared light-triggered drug release, Nanoscale, 2015, 7, 19092–19098 RSC.
- M. Liouni, P. Drichoutis and E. T. Nerantzis, Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads, using Saccharomyces cerevisiae entrapped cells, World J. Microbiol. Biotechnol., 2008, 24, 281–288 CrossRef CAS.
- J. Wang, K. Van Tittelboom, N. De Belie and W. Verstraete, Use of silica gel or polyurethane immobilized bacteria for self-healing concrete, Constr. Build. Mater., 2012, 26, 532–540 CrossRef.
- J. Mi, E. G. Gregorich, S. Xu, N. B. McLaughlin, B. Ma and J. Liu, Effect of bentonite amendment on soil hydraulic parameters and millet crop performance in a semi-arid region, Field Crops Res., 2017, 212, 107–114 CrossRef.
- A. S. Liffourrena and G. I. Lucchesi, Alginate-perlite encapsulated Pseudomonas putida A (ATCC 12633) cells: Preparation, characterization and potential use as plant inoculants, J. Biotechnol., 2018, 278, 28–33 CrossRef CAS PubMed.
- N. Vassilev, M. Vassileva, R. Azcon and A. Medina, Application of free and Ca-alginate-entrapped glomus deserticola and Yarowia lipolytica in a soil–plant system, J. Biotechnol., 2001, 91, 237–242 CrossRef CAS PubMed.
- M. S. Arif, S. M. Shahzad, M. Riaz, T. Yasmeen, T. Shahzad, M. J. Akhtar, L. Bragazza and A. Buttler, Nitrogen-enriched compost application combined with plant growth-promoting rhizobacteria (PGPR) improves seed quality and nutrient use efficiency of sunflower, J. Plant Nutr. Soil Sci., 2017, 180, 464–473 CrossRef CAS.
- R. Prasad, M. Kumar and A. Varma, in Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants, ed. D. Egamberdieva, S. Shrivastava and A. Varma, Springer International Publishing, Cham, 2015, DOI:10.1007/978-3-319-13401-7_12, pp. 247–260.
- J. Yadav, J. P. Verma, D. K. Jaiswal and A. Kumar, Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa), Ecol. Eng., 2014, 62, 123–128 CrossRef.
- S. A. Strobel, K. Allen, C. Roberts, D. Jimenez, H. B. Scher and T. Jeoh, Industrially-scalable microencapsulation of plant beneficial bacteria in dry cross-linked alginate matrix, Ind. Biotechnol., 2018, 14, 138–147 CrossRef CAS PubMed.
- Y. H. He, Z. S. Wu, W. F. Wang, X. C. Liu and B.-C. Ye, Bacterial community and phosphorus species changes in pepper rhizosphere soils after Pseudomonas putida Rs-198 inoculation, Rhizosphere, 2019, 11, 100164 CrossRef.
- F. Deng, C. Liao, C. Yang, C. Guo, L. Ma and Z. Dang, A new approach for pyrene bioremediation using bacteria immobilized in layer-by-layer assembled microcapsules: dynamics of soil bacterial community, RSC Adv., 2016, 6, 20654–20663 RSC.
- X. Song, M. Liu, D. Wu, B. S. Griffiths, J. Jiao, H. Li and F. Hu, Interaction matters: Synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field, Appl. Soil Ecol., 2015, 89, 25–34 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |