Open Access Article
Open Access ArticleC–O/C–S difunctionalized benzene derivatives via multicomponent coupling of tetraynes†
Liangliang
Yao
,
Qiong
Hu
,
Yu
Lei
,
Li
Bao
and
Yimin
Hu
 *
*
Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Key Laboratory of Molecular-Based Materials, School of Chemistry and Materials Science, Anhui Normal University, Wuhu, Anhui 241000, China. E-mail: yiminhu@ahnu.edu.cn
First published on 28th September 2020
Abstract
C–O/C–S difunctionalization of fused highly substituted benzene derivatives was conducted via the multicomponent coupling reaction of tetraynes, sulfoxides, and cyclopropenones. This reaction is associated with several bond cleavage and formation reactions in one pot, and also features exquisite regioselectivity and excellent yields. Preliminary mechanistic studies reveal that the reaction possibly proceeds in a sequential [2 + 2] cycloaddition, ring-opening of the cyclopropenone, protonation and dealkylation pathway.
Carbon–heteroatom bond formation is one of the most important tools used in pharmaceutical chemistry, medicinal chemistry, and materials sciences.1 One-step synthesis of Csp2–O and Csp2–S bonds is an important and challenging task for chemists. Xiao and Chen disclosed the first case of aryne insertion into the S
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of sulfoxide for the direct synthesis of 1,2-O,S-disubstituted arenes.2 Wang's group developed a synthesis technique for ortho-aryloxydiaryl sulfides in the absence of an external electrophile.3 Li and co-workers reported tandem aryne S
O bond of sulfoxide for the direct synthesis of 1,2-O,S-disubstituted arenes.2 Wang's group developed a synthesis technique for ortho-aryloxydiaryl sulfides in the absence of an external electrophile.3 Li and co-workers reported tandem aryne S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond insertion/C–H functionalization, featuring arene 1,2,3-trisubstitution from a relatively simple aryl allyl sulfoxide.4 Peng and co-workers synthesized o-aryloxytriaryl sulfonium salts instead of thioethers via aryne insertion into diaryl sulfoxides.5 Studer and his group designed a new protocol for the direct synthesis of o-arylsulfinyl aryl vinyl ethers by using aryne and aryl vinyl sulfoxides via aryne insertion into the S–O σ-bond and concomitant stereospecific S–O vinyl migration.6 Gogoi's group designed an interesting three component reaction for 2-[(o-methylthio)aryloxy] substituted dialkyl maleate derivatives by using aryne, dimethyl sulfoxide and activated alkynes.7 Many o-oxygen substituted aryl sulfides have been synthesized via aryne insertion into the S
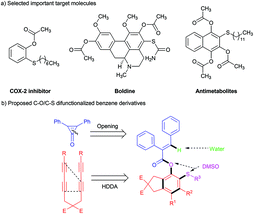
O bond insertion/C–H functionalization, featuring arene 1,2,3-trisubstitution from a relatively simple aryl allyl sulfoxide.4 Peng and co-workers synthesized o-aryloxytriaryl sulfonium salts instead of thioethers via aryne insertion into diaryl sulfoxides.5 Studer and his group designed a new protocol for the direct synthesis of o-arylsulfinyl aryl vinyl ethers by using aryne and aryl vinyl sulfoxides via aryne insertion into the S–O σ-bond and concomitant stereospecific S–O vinyl migration.6 Gogoi's group designed an interesting three component reaction for 2-[(o-methylthio)aryloxy] substituted dialkyl maleate derivatives by using aryne, dimethyl sulfoxide and activated alkynes.7 Many o-oxygen substituted aryl sulfides have been synthesized via aryne insertion into the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of sulfoxides. Two or more substituted o-alkylthio aryl esters are the important structural units in biologically active molecules and medicines, such as cox-2 inhibitors,8 boldine,9 and antimetabolites10 (Scheme 1a). Therefore, an efficient strategy is desirable to be developed for the C–O/C–S difunctionalization of fused highly substituted benzene derivatives.
O bond of sulfoxides. Two or more substituted o-alkylthio aryl esters are the important structural units in biologically active molecules and medicines, such as cox-2 inhibitors,8 boldine,9 and antimetabolites10 (Scheme 1a). Therefore, an efficient strategy is desirable to be developed for the C–O/C–S difunctionalization of fused highly substituted benzene derivatives.
Demonstrated by Hoye's group in 2012,11 the hexadehydro-Diels–Alder (HDDA) reaction, a new strategy to produce benzyne intermediates, can assemble three consecutive functional groups on a benzene ring in a “one-pot” process, and is compatible with various reagents and functional groups.12 These benzyne intermediates are suitable for versatile transformations, thereby greatly expanding the fields of aryne chemistry and drug synthesis.13 As part of our ongoing research on the efficient construction of polysubstituted arenes via HDDA-derived benzyne chemistry,14 we envisioned that different tetrayne substrates can be used as HDDA-derived benzyne precursors to form the C–O/C–S difunctionalized and concomitant o-alkylthio aryl ester core structure benzene derivatives by inserting benzyne into the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and opening the ring of cyclopropenone (Scheme 1b). To the best of our knowledge, this hypothesis has not been verified by using benzyne intermediates. Thus, exploring the resultant intermediate could provide a novel platform to incorporate sulfide and ester groups into valuable aromatic compounds.
O bond and opening the ring of cyclopropenone (Scheme 1b). To the best of our knowledge, this hypothesis has not been verified by using benzyne intermediates. Thus, exploring the resultant intermediate could provide a novel platform to incorporate sulfide and ester groups into valuable aromatic compounds.
A multicomponent coupling reaction of HDDA-derived benzynes, cyclopropenones, and sulfoxides was performed to produce various C–O/C–S difunctionalized benzene derivatives in good to excellent yields. The process of introducing sulfide and ester moieties into an aromatic ring can be achieved in the absence of catalysts, bases, or oxidants and therefore could provide a broad range of construction methods for fully functionalized arenes. Synthesis chemists can achieve the rapid synthesis of drug molecules and other high-value compounds.
Results and discussion
Inspired by the preliminary result, tetrayne 1a was used as a HDDA-derived benzyne precursor and 2,3-diphenylcycloprop-2-enone 2a in DMSO at 90 °C to optimize the reaction conditions. Screening results revealed that the optimal yield could be obtained at 120 °C in DMSO at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 ratio of tetrayne to cyclopropenone in an 8 h reaction. It was verified that DMSO played a crucial role in this multicomponent coupling reaction. The optimal conditions were as follows: 1.0 equiv. of tetraynes reacted with 1.1 equiv. of cyclopropenones in DMSO at 120 °C for 8 h.
1.1 ratio of tetrayne to cyclopropenone in an 8 h reaction. It was verified that DMSO played a crucial role in this multicomponent coupling reaction. The optimal conditions were as follows: 1.0 equiv. of tetraynes reacted with 1.1 equiv. of cyclopropenones in DMSO at 120 °C for 8 h.
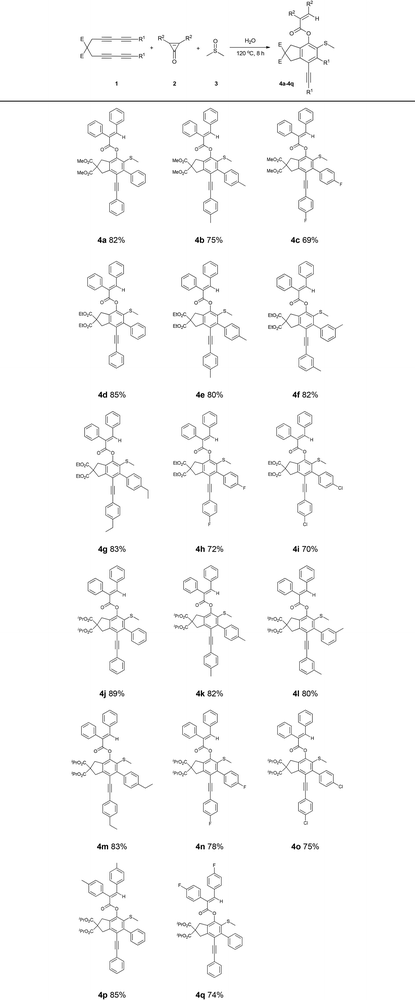
The scope of tetrayne substrates was investigated under the optimized conditions. As depicted in Table 1, various products (4a–4q) were readily isolated with good to excellent yields (ranging from 69% to 89%) from the reactions of tetraynes 1 with 2,3-diphenylcycloprop-2-enone 2a in DMSO. The effect of different tetraynes on the product yield was also examined. When tolerated, tetrayne substrates bearing different esters (OMe, OEt, and OiPr) gradually increased in yields (4a (82%), 4d (85%), and 4j (89%)). By contrast, the substituent groups in the aryl rings of tetraynes bearing electron-donating groups, including p-Me, m-Me, and p-Et (4k, 4l, and 4m), exhibited higher yields than the electron-withdrawing groups, such as p-F and p-Cl (4n and 4o) possibly because the electron-withdrawing groups decreased the reactivity. Compound 4j had the highest isolated yield (89%) among the examined products.
Furthermore, 2,3-di-p-tolylcycloprop-2-enone and 2,3-bis(4-fluorophenyl)cycloprop-2-enone can participate in the reaction to afford good yields (4p (85%) and 4q (74%)). The structures of 4a and 4m were confirmed through X-ray diffraction.15
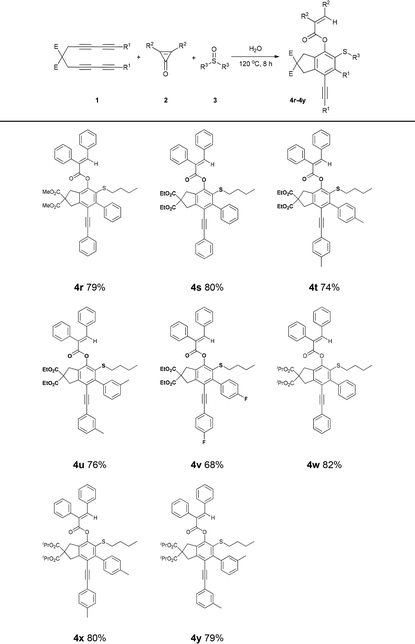
As shown in Table 2, 68%–82% yields were obtained from the reactions of tetraynes 1 with 2,3-diphenylcycloprop-2-enone 2a in n-butyl sulfoxide. The carboxylates of the tetraynes with OiPr, OEt and OMe provided minimal impact, providing 4r (79%), 4s (80%), and 4w (82%), respectively. The yields of benzene ring-containing tetraynes with alkyl groups as electron donors were slightly higher than those of tetraynes directly bearing halogen atoms as electron-withdrawing groups. For example, the yields of 4t and 4u were 74% and 76%, respectively, and that of 4v was only 68%. These results revealed the potential of the multicomponent coupling reaction of current HDDA-derived benzynes with cyclopropenones and sulfoxides for the synthesis of C–O/C–S difunctionalized benzene derivatives.
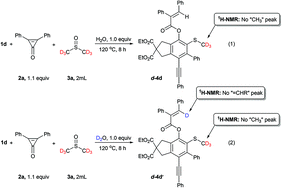
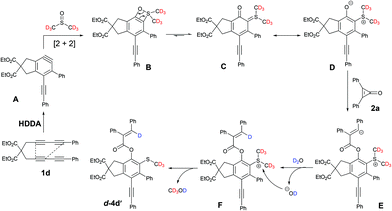
Labeling and control experiments were performed to gain insight into the reaction mechanism (Scheme 2). DMSO-d6 was used instead of DMSO for the synthesis of 4d under the optimized reaction conditions (Scheme 2, eqn (1)). The deuterium-labeled product d-4d was isolated in 83% yield. 1H NMR analysis confirmed the complete deuterium incorporation at the “CH3” moiety, indicating that DMSO serves as the “SMe” source. Another independent experiment was performed in which D2O was used instead of H2O with 1d, and 2a in DMSO-d6 to further investigate the reaction mechanism (Scheme 2, eqn (2)). The expected product d-4d′ was formed in 84% yield with complete deuterium incorporation at the vinylic position. These results suggest that our reaction proceeded via the [2 + 2] cycloaddition of a HDDA-derived benzyne with DMSO solvent, and that H2O served as the proton source (details are in the Experimental section and ESI†).
A possible mechanism underlying the formation of C–O/C–S difunctionalized benzene derivatives was proposed on the basis of the above results and previous literature2,7,16 (Scheme 3). First, the HDDA-derived benzyne intermediate A underwent [2 + 2] cycloaddition insertion into the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of dimethyl sulfoxide to form a four-membered ring intermediate B. Owing to the ring strain, this intermediate exhibited ring opening to afford an o-quinone intermediate C. Zwitterion D, a resonance form of C, also experienced nucleophilic attack and ring opening to activate the cycolpropenone 2a. This phenomenon leads to the formation of zwitterion E, which was further protonated in the presence of residual water to form intermediate F. Finally, the resultant hydroxide anion from H2O abstracted the methyl cation of intermediate F to generate the product d-4d′.
O bond of dimethyl sulfoxide to form a four-membered ring intermediate B. Owing to the ring strain, this intermediate exhibited ring opening to afford an o-quinone intermediate C. Zwitterion D, a resonance form of C, also experienced nucleophilic attack and ring opening to activate the cycolpropenone 2a. This phenomenon leads to the formation of zwitterion E, which was further protonated in the presence of residual water to form intermediate F. Finally, the resultant hydroxide anion from H2O abstracted the methyl cation of intermediate F to generate the product d-4d′.
Conclusions
In conclusion, we have developed a multicomponent coupling reaction of HDDA-derived benzynes, cyclopropenones and sulfoxides for the direct synthesis of C–O/C–S difunctionalized benzene derivatives. This reaction strategy has been accomplished through several bond cleavage and formation reactions in a one-pot procedure. Compared with previous work, this strategy introduces sulfide and ester moieties at the adjacent positions of an arene ring with the C–O and C–S bonds and derivatives thereof. The reaction does not require a transition metal catalyst but exhibits excellent regioselectivity and produces all types of highly substituted fused benzene derivatives under mild conditions with good to excellent yields. Further work on the applications and scope extension of this protocol is on-going in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (22071001, 21572002) and the Department of Human Resources of Anhui Province for financial support.Notes and references
- (a) Catalyzed Carbon–Heteroatom Bond Formation, ed. A. K. Yudin, Wiley-VCH, Weinheim, 2011 Search PubMed; (b) J. Yin, in Applications of Transition Metal Catalysis in Drug Discovery and Development, ed. M. L. Crawley and B. M. Trost, John Wiley and Sons, Hoboken, 2012, pp. 97–163 Search PubMed; (c) A. Bhunia, S. R. Yetra and A. T. Biju, Recent advances in transition-metal-free carbon–carbon and carbon–heteroatom bond-forming reactions using arynes, Chem. Soc. Rev., 2012, 41, 3140–3152 RSC; (d) X. Li, X. Li, M. N. Banis, B. Wang, A. Lushington, X. Cui, R. Li, T.-K. Sham and X. Sun, Tailoring interactions of carbon and sulfur in Li–S battery cathodes: significant effects of carbon–heteroatom bonds, J. Mater. Chem. A, 2014, 2, 12866–12872 RSC; (e) H.-T. Kim, H. Shin, I.-Y. Jeon, M. Yousaf, J. Baik, H.-W. Cheong, N. Park, J.-B. Baek and T.-H. Kwon, Carbon–heteroatom bond formation by an ultrasonic chemical reaction for energy storage systems, Adv. Mater., 2017, 29, 1702747 CrossRef.
- F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Three-component coupling reaction triggered by insertion of arynes into the S=O bond of DMSO, Org. Lett., 2014, 16, 3768–3771 CrossRef CAS.
- H.-Y. Li, L.-J. Xing, M.-M. Lou, H. Wang, R.-H. Liu and B. Wang, Reaction of arynes with sulfoxides, Org. Lett., 2015, 17, 1098–1101 CrossRef CAS.
- Y. Li, D. Qiu, R. Gu, J. Wang, J. Shi and Y. Li, Aryne 1,2,3-trifunctionalization with aryl allyl sulfoxides, J. Am. Chem. Soc., 2016, 138, 10814–10817 CrossRef CAS.
- X. Li, Y. Sun, X. Huang, L. Zhang, L. Kong and B. Peng, Synthesis of o-aryloxy triarylsulfonium salts via aryne insertion into diaryl sulfoxides, Org. Lett., 2017, 19, 838–841 CrossRef CAS.
- Y. Li and A. Studer, Reaction of arynes with vinyl sulfoxides: Highly stereospecific synthesis of ortho-sulfinylaryl vinyl ethers, Org. Lett., 2017, 19, 666–669 CrossRef CAS.
- H. Hazarika, K. Neog, A. Sharma, B. Das and P. Gogoi, Three-component coupling reactions of aryne, DMSO, and activated alkyne: stereoselective synthesis of 2-[(o-methylthio)aryloxy]-substituted dialkyl maleates, J. Org. Chem., 2019, 84, 5846–5854 CrossRef CAS.
- (a) A. S. Kalgutkar, B. C. Crews, S. W. Rowlinson, C. Garner, K. Seibert and L. J. Marnet, Aspirin-like molecules that covalently inactivate cyclooxygenase-2, Science, 1998, 280, 1268–1270 CrossRef CAS; (b) A. S. Kalgutkar, K. R. Kozak, B. C. Crews, G. P. Hochgesang Jr. and L. J. Marnett, Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators, J. Med. Chem., 1998, 41, 4800–4818 CrossRef CAS; (c) C. F. Pereira, J. T. M. L. Paridaen, K. Rutten, M. C. D. G. Huigen, M. van de Bovenkamp, J. Middel, N. Beerens, B. Berkhout, R. Schuurman, L. J. Marnett, J. Verhoef and H. S. L. M. Nottet, Aspirin-like molecules that inhibit human immunodeficiency virus 1 replication, Antiviral Res., 2003, 58, 253–263 CrossRef CAS; (d) H. K. Jain, V. K. Mouryab and R. K. Agrawal, Inhibitory mode of 2-acetoxyphenyl alkyl sulfides against COX-1 and COX-2: QSAR analyses, Bioorg. Med. Chem. Lett.,, 2006, 16, 5280–5284 CrossRef CAS.
- F. A. Thometa, P. Pinyolb, J. Villenab, L. J. Espinozaa and P. G. Reveco, Cytotoxic thiocarbamate derivatives of boldine, Nat. Prod. Commun., 2010, 5, 1587–1590 Search PubMed.
- T. H. Porter, C. M. Bowman and K. Folkers, Antimetabolites of coenzyme Q. 16. New alkylmercaptoquinones having antimalarial curative activity, J. Med. Chem., 1973, 16, 115–118 CrossRef CAS.
- T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, The hexadehydro-Diels–Alder reaction, Nature, 2012, 490, 208–212 CrossRef CAS.
- For selected reviews and examples, see: (a) T. R. Hoye, B. Baire and T. Wang, Tactics for probing aryne reactivity: mechanistic studies of silicon–oxygen bond cleavage during the trapping of (HDDA-generated) benzynes by silyl ethers, Chem. Sci., 2014, 5, 545–550 RSC; (b) R. Karmakar and D. Lee, Reactions of arynes promoted by silver ions, Chem. Soc. Rev., 2016, 45, 4459–4470 RSC; (c) X. Xiao, T. Wang, F. Xu and T. R. Hoye, CuI-mediated bromoalkynylation and hydroalkynylation reactions of unsymmetrical benzynes: Complementary modes of addition, Angew. Chem., Int. Ed., 2018, 57, 16564–16568 CrossRef CAS; (d) S. Gupta, Y. Lin, Y. Xia, D. J. Winka and D. Lee, Alder-ene reactions driven by high steric strain and bond angle distortion to form benzocyclobutenes, Chem. Sci., 2019, 10, 2212–2217 RSC; (e) S. Arora, J. Zhang, V. Pogula and T. R. Hoye, Reactions of thermally generated benzynes with six-membered N-heteroaromatics: pathway and product diversity, Chem. Sci., 2019, 10, 9069–9076 RSC; (f) S. Ghorai, Y. Lin, Y. Xia, D. J. Wink and D. Lee, Silver-catalyzed annulation of arynes with nitriles for synthesis of structurally diverse quinazolines, Org. Lett., 2020, 22, 626–630 CrossRef CAS.
- For selected examples, see: (a) D. Niu and T. R. Hoye, The aromatic ene reaction, Nat. Chem., 2013, 6, 34–40 CrossRef; (b) T. Wang and T. R. Hoye, Hexadehydro-Diels−Alder (HDDA)-enabled carbazolyne chemistry: Single step, de novo construction of the pyranocarbazole core of alkaloids of the Murraya koenigii (Curry tree) family, J. Am. Chem. Soc., 2016, 138, 13870–13873 CrossRef CAS; (c) R. Karmakar and D. Lee, Total synthesis of Selaginpulvilin C and D relying on in situ formation of arynes and their hydrogenation, Org. Lett., 2016, 18, 6105–6107 CrossRef CAS; (d) S. P. Ross and T. R. Hoye, Reactions of hexadehydro-Diels–Alder benzynes with structurally complex multifunctional natural products, Nat. Chem., 2017, 9, 523–530 CrossRef CAS; (e) X. Xiao and T. R. Hoye, The domino hexadehydro-Diels–Alder reaction transforms polyynes to benzynes to naphthynes to anthracynes to tetracynes (and beyond?), Nat. Chem., 2018, 10, 838–844 CrossRef CAS; (f) X. Xiao and T. R. Hoye, One-pot, three-aryne cascade strategy for naphthalene formation from 1,3-diynes and 1,2-benzdiyne equivalents, J. Am. Chem. Soc., 2019, 141, 9813–9818 CrossRef CAS.
- (a) Y. Hu, J. Ma, L. Li, Q. Hu, S. Lv, B. Liu and S. Wang, Fused multifunctionalized dibenzoselenophenes from tetraynes, Chem. Commun., 2017, 53, 1542–1545 RSC; (b) B. Liu, C. Mao, Q. Hu, L. Yao and Y. Hu, Direct methylation and carbonylation of in situ generated arynes via HDDA-Wittig coupling, Org. Chem. Front., 2019, 6, 2788–2791 RSC; (c) B. Liu, Q. Hu, F. Yang, X. Zheng and Y. Hu, Fused multifunctionalized bridge aromatic hydrocarbons from in situ-generated arynes and anthracene derivatives, Chin. Chem. Lett., 2020, 31, 1305–1308 CrossRef CAS; (d) X. Zheng, B. Liu, F. Yang, Q. Hu, L. Yao and Y. Hu, Access to benzoxazepines and fully substituted indoles via HDDA Coupling, Org. Lett., 2020, 22, 956–959 CrossRef CAS.
- CCDC 1571270 (4a) and 1571269 (4m) contain the supplementary crystallographic data for this paper.†.
- H. Hazarikaa and P. Gogoi, Direct synthesis of ortho-methylthio allyl and vinyl ethers via three component reaction of aryne, activated alkene and DMSO, Org. Biomol. Chem., 2020, 18, 2727–2738 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of all new compounds. CCDC 1571269–1571270. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qo00967a |
| This journal is © the Partner Organisations 2020 |