Indolizine synthesis via copper-catalyzed cyclization of gem-difluoroalkenes and 2-(pyridin-2-yl)acetate derivatives†
Abstract
A novel and versatile approach to construct substituted indolizines through copper-catalyzed coupling cyclization of 2-(pyridin-2-yl)acetate with gem-difluoroalkenes has been developed. This method takes advantage of the cleavage of C–F bonds, providing a straightforward and efficient access to a variety of bisubstituted indolizine derivatives in moderate to good yields with brilliant functional group compatibility.
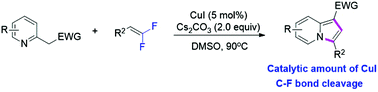


 Please wait while we load your content...
Please wait while we load your content...