Dual N-heterocyclic carbene/photocatalysis: a new strategy for radical processes
Qiang
Liu
and
Xiang-Yu
Chen
 *
*
School of Chemical Sciences, University of the Chinese Academy of Sciences, Beijing 100049, China. E-mail: chen.xiangyu@rwth-aachen.de
First published on 23rd June 2020
Abstract
While N-heterocyclic carbenes (NHCs) have played an important role as Lewis-base organocatalysts and developed recently into a full chapter of this exciting area of organocatalysis, NHC-catalyzed single-electron-transfer (SET) processes are still challenging and underdeveloped. Recently, NHC catalysis has been successfully combined with photoredox catalysis for SET reactions and this turned out to be a promising strategy. This article focuses on the recent advances of the combination of photoredox catalysis with NHC catalysis.
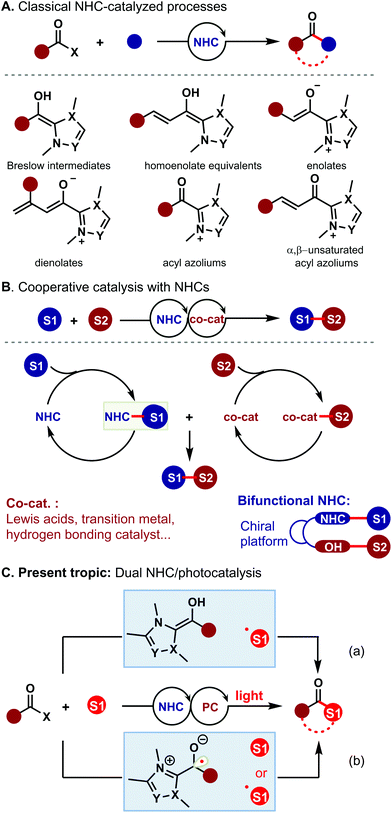
In the past decades, N-heterocyclic carbenes (NHCs) have witnessed remarkable achievements, and they are complementary to other organocatalysts and enable the creation of new activation modes that were previously unobtainable.1 A driving force is the great variety of reactive intermediates that can be generated with NHCs (Scheme 1A). Despite this progress, most of these transformations proceed via electron-pair-transfer pathways; the corresponding reactions via single-electron-transfer (SET) processes remain challenging.2
In recent years, cooperative catalysis with NHCs has remarkably improved the reaction efficiency and selectivity that are unobtainable by using single-catalyst systems (Scheme 1B). Interestingly, cooperative NHC/Lewis acid catalysis has strongly dominated this field,4 with comparatively less synergistic combination of NHC catalysis with other types of catalysis reported in the literature.3–5 Recently, there has been growing interest in harnessing the synergistic interplay of NHCs and photochemistry to develop new activation modes that provide more efficient and selective alternatives to classical approaches. An early example of dual NHC/photocatalysis was reported by Rovis and co-workers.6 In their system, the photocatalyst Ru(bpy)3Cl2 and NHC were employed for the α-acylation of tertiary amines. In 2016, an NHC/photo co-catalyzed γ-dichloromethylenation of enal was reported by Sun and co-workers.2g Although these examples have shown the synthetic utility of dual NHC/photocatalysis, further development of this strategy to create new activation modes is still highly desirable. This manuscript aims to provide a brief summary of light-driven NHC-catalyzed reactions and highlight recent publications in this area.
At present, there are two types of reaction modes associated with dual NHC/photocatalysis. One is the combination of classical NHC-intermediates and the generated radicals via photocatalysis. The other is the use of a new kind of NHC-derived radical intermediate via photocatalysis (Scheme 1C).
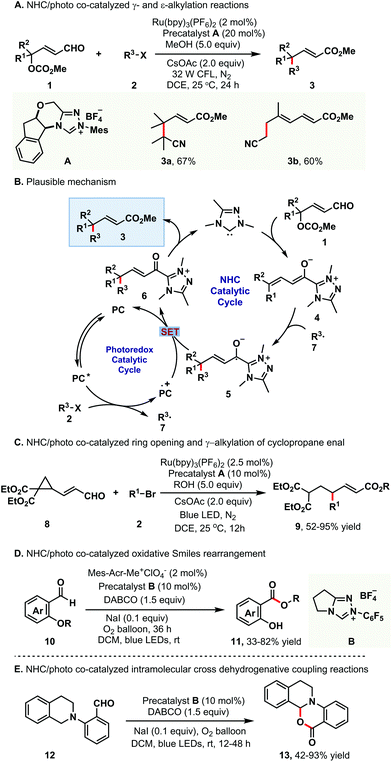
Recently, Ye and co-workers have made important contributions to dual NHC/photocatalysis by using reaction mode a. They successfully realized the γ- and ε-alkylation of enals via dual NHC/photocatalysis, offering direct access to γ- and ε-alkylated esters 3 in very good yields.7 Notably, the challenging vicinal all-carbon quaternary centers could be efficiently constructed by using this strategy (Scheme 2A). The key step of this transformation was the formation of an alkyl radical via photocatalysis and a dienolate/trienolate intermediate via NHC catalysis. To investigate the mechanism of this alkylation reaction, several control experiments, such as radical trapping and radical clock experiments, were carried out. The plausible mechanism is proposed in Scheme 2B. The alkyl radical 7 is generated via photocatalysis from alkyl halide while the dienolate intermediate 4 is generated from enal 1via NHC catalysis. The alkyl radical 7 reacts with the dienolate intermediate 4 to afford the homoenolate radical 5, which undergoes single-electron transfer (SET) oxidation with the radical cation of the photocatalyst to give α,β-unsaturated acyl azolium intermediate 6. Lastly, the acyl azolium intermediate 6 is trapped by methanol to afford the corresponding product 3 and liberate the NHC catalyst.
Subsequently, the same group further expanded this strategy to the ring-opening and γ-alkylation of cyclopropane enal (Scheme 2C).8 This method provided an efficient access to γ-alkylated α,β-unsaturated esters 9. A series of alcohols and brominated compounds 2 reacted smoothly to deliver the target products 9 in 52–95% yields. Unfortunately, other enals, such as epoxy enals and several other cyclopropane enals, failed to couple with brominated compounds. The authors proposed that the dienolate intermediate could be generated from γ-cyclopropane enal through NHC-catalyzed C–C bond cleavage, which was in agreement with Zhao's pathway.9 The following similar steps to those in Scheme 2B afforded the product 9 and closed the catalytic cycle.
Apart from the above-mentioned contributions, Ye and co-workers also developed the oxidative Smiles rearrangement reaction via dual NHC/photocatalysis (Scheme 2D).10 Here, O-aryl salicylaldehydes were transformed into aryl salicylates in the presence of oxygen as the oxidant. This study revealed that the oxidation of the Breslow intermediate was achieved by oxygen with acridinium and NaI as the (co)catalysts and offered a new methodology for oxidative NHC catalysis. Later, this strategy was successfully extended to the intramolecular cross-dehydrogenative coupling of tetrahydroisoquinoline-tethered aldehydes (Scheme 2E).11 Notably, this photoinduced oxidative reaction worked well without an external photocatalyst and an excited Breslow intermediate facilitated the photooxidation course.
Simultaneously, Hopkinson and co-workers developed a novel protocol for the Diels–Alder reaction of acid fluorides with trifluoromethyl ketones via reaction mode b (Scheme 3A).12 In their system, 1,3-dimethylimidazolium triflate C as the precatalyst and Cs2CO3 as the base were employed under the irradiation of UVA LEDs. A range of acid fluorides 14 and trifluoromethyl ketones 15 were investigated to verify the wide substrate scope tolerance. Unfortunately, other classes of nonenolizable ketones, such as 2-ketoesters and isatins, didn't work under the current conditions. To gain insight into the reaction mechanism, stoichiometric studies and time-dependent DFT calculations were conducted to support the mechanistic scenario where the combination of the NHC and the acid fluoride led to an interim change in the absorption properties and photochemical reactivity of the carbonyl function. The proposed mechanism is shown in Scheme 3B; the in situ generated NHC reacts with acid fluoride 14a to deliver the o-toluoyl azolium intermediate 17 and releases F−. This azolium intermediate 17 can be excited under UVA irradiation and provides the triplet excited state intermediate 18 after intersystem crossing. Rapid 1,5-H abstraction from the o-benzylic position to the radical-like carbonyl oxygen produces the triplet dienol biradical 19. Rotation of this biradical before relaxation results in the ground state 20, which reacts with the trifluoromethyl ketone 15a in a (4 + 2) cycloaddition process to afford the cycloadduct 21. Finally, elimination of the NHC in the presence of the base gives the desired product 16a and completes the catalytic cycle.
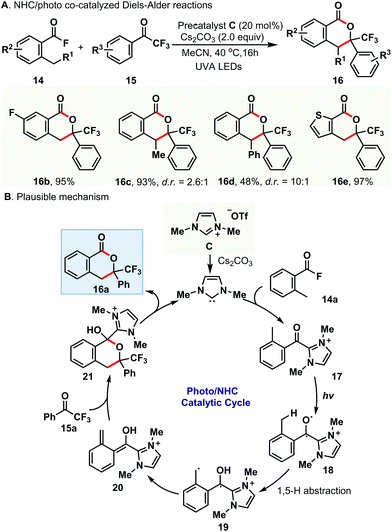 | ||
| Scheme 3 NHC/photo co-catalyzed Diels–Alder reaction of acid fluorides with trifluoromethyl ketones via reaction mode b. | ||
Most recently, another elegant example of combining photocatalysis with NHC catalysis has been demonstrated by Scheidt and co-workers.13 They developed a novel NHC/photo co-catalyzed alkylation reaction between acyl imidazoles 22 and Hantzsch esters 23 for the efficient synthesis of ketones 24 (Scheme 4A). This method enabled the coupling of an acyl radical with an alkyl radical by using the triazolium salt D as the precatalyst and iridium photocatalyst. To further demonstrate the utility of this method, various pharmaceutical carboxylic acid drugs, such as telmisartan, repaglinide and dehydrocholic acid, were applied for the one-step direct alkylation reaction to afford the corresponding ketone products in moderate to good yields. A series of control reactions and radical-trapping experiments were performed, which indicated that the oxidation of the Hantzsch ester occurred prior to the reduction of the acyl azolium. The proposed reaction pathway is shown in Scheme 4B. The Hantzsch ester 23a is oxidized to generate the radical cation 25 using the photoexcited catalyst (IrIII*); then cleavage of the radical cation 25 provides the benzyl radical. The following reduction of the acyl triazolium intermediate 26via IrII gives the crucial azolium radical 27. The final coupling of the benzyl radical and the radical intermediate 28 affords the desired ketone 24c and liberates the NHC catalyst.
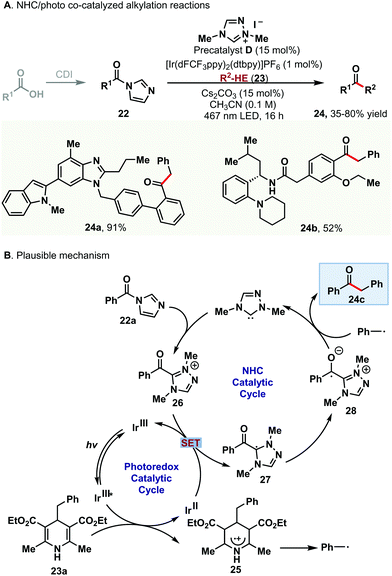 | ||
| Scheme 4 NHC/photo co-catalyzed alkylation of acyl imidazoles with Hantzsch esters via reaction mode b. | ||
In summary, as demonstrated by the examples discussed, dual NHC/photocatalysis will lead to the development of a wide range of novel transformations with broad synthetic applications. This methodology has shown its potential advantage of introducing radicals into NHC catalysis. Although the NHC/photo co-catalyzed reaction is now at the stage of infancy, these studies have impressively illustrated that combining photocatalysis and NHC catalysis is a prospective strategy. In future research, several challenges need to be addressed; a wide range of NHC derived intermediates—especially homoenolate equivalents and α,β-unsaturated acyl azolium intermediates—should be investigated, and compared with well-developed NHC-catalyzed asymmetric reactions, the development of enantioselective approaches is still needed.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The School of Chemical Sciences, University of the Chinese Academy of Sciences is gratefully acknowledged.Notes and references
- For selected recent reviews on NHC catalysis, see: (a) D. Enders, O. Niemeier and A. Henseler, Organocatalysis by N-Heterocyclic Carbenes, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) A. Grossmann and D. Enders, N-Heterocyclic Carbene Catalyzed Domino Reactions, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (c) J. W. Bode, Carbene Catalysis: an Internal Affair, Nat. Chem., 2013, 5, 813 CrossRef CAS PubMed; (d) X. Bugaut and F. Glorius, Organocatalytic umpolung: N-heterocyclic carbenes and beyond, Chem. Soc. Rev., 2012, 41, 3511 RSC; (e) S. De.Sarkar, A. Biswas, R. C. Samanta and A. Studer, Catalysis with N-Heterocyclic Carbenes under Oxidative Conditions, Chem. – Eur. J., 2013, 19, 4664 CrossRef CAS PubMed; (f) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, An Overview of N-Heterocyclic Carbenes, Nature, 2014, 510, 485 CrossRef CAS PubMed; (g) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed; (h) R. S. Menon, A. T. Biju and V. Nair, Recent Advances in Employing Homoenolates Generated by N-Heterocyclic Carbene (NHC) Catalysis in Carbon-Carbon Bond-Forming Reactions, Chem. Soc. Rev., 2015, 44, 5040 RSC; (i) C. Zhang, J. F. Hooper and D. W. Lupton, N-Heterocyclic Carbene Catalysis via the α,β-Unsaturated Acyl Azolium, ACS Catal., 2017, 7, 2583 CrossRef CAS; (j) K. J. R. Murauski, A. A. Jaworski and K. A. Scheidt, A Continuing Challenge: N-Heterocyclic Carbene-Catalyzed Syntheses of γ-Butyrolactones, Chem. Soc. Rev., 2018, 47, 1773 RSC; (k) X.-Y. Chen, Q. Liu, P. Chauhan and D. Enders, N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations, Angew. Chem., Int. Ed., 2018, 57, 3862 CrossRef CAS PubMed; (l) S. Mondal, S. R. Yetra, S. Mukherjee and A. T. Biju, NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles, Acc. Chem. Res., 2019, 52, 425 CrossRef CAS PubMed; (m) X.-Y. Chen, Z.-H. Gao and S. Ye, Bifunctional N-Heterocyclic Carbenes Derived from L-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis, Acc. Chem. Res., 2020, 53, 690 CrossRef CAS PubMed.
- (a) J. Guin, S. De Sarkar, S. Grimme and A. Studer, Biomimetic Carbene-Catalyzed Oxidations of Aldehydes Using TEMPO, Angew. Chem., Int. Ed., 2008, 47, 8727 CrossRef CAS PubMed; (b) Y. Du, Y. Wang, X. Li, Y. Shao, G. Li, R. D. Webster and Y. R. Chi, N-Heterocyclic Carbene Organocatalytic Reductive β,β-Coupling Reactions of Nitroalkenes via Radical Intermediates, Org. Lett., 2014, 16, 5678 CrossRef CAS PubMed; (c) N. A. White and T. Rovis, Enantioselective N-Heterocyclic Carbene-Catalyzed β-Hydroxylation of Enals Using Nitroarenes: An Atom Transfer Reaction That Proceeds via Single Electron Transfer, J. Am. Chem. Soc., 2014, 136, 14674 CrossRef CAS PubMed; (d) N. A. White and T. Rovis, Oxidatively Initiated NHC-Catalyzed Enantioselective Synthesis of 3,4-Disubstituted Cyclopentanones from Enals, J. Am. Chem. Soc., 2015, 137, 10112 CrossRef CAS PubMed; (e) Y. Zhang, Y. Du, Z. Huang, J. Xu, X. Wu, Y. Wang, M. Wang, S. Yang, R. D. Webster and Y. R. Chi, N-Heterocyclic Carbene-Catalyzed Radical Reactions for Highly Enantioselective β-Hydroxylation of Enals, J. Am. Chem. Soc., 2015, 137, 2416 CrossRef CAS PubMed; (f) J. Rehbein, S.-M. Ruser and J. Phan, NHC-Catalysed Benzoin Condensation–is it all down to the Breslow Intermediate?, Chem. Sci., 2015, 6, 6013 RSC; (g) W. Yang, W. Hu, X. Dong, X. Li and J. Sun, N-Heterocyclic Carbene Catalyzed γ-Dihalomethylenation of Enals by Single-Electron Transfer, Angew. Chem., Int. Ed., 2016, 55, 15783 CrossRef CAS PubMed; (h) B.-S. Li, Y. Wang, R. S. J. Proctor, Y. Zhang, R. D. Webster, S. Yang, B.-A. Song and Y. R. Chi, Carbene-Catalysed Reductive Coupling of Nitrobenzyl Bromides and Activated Ketones or Imines via Single-Electron-Transfer Process, Nat. Commun., 2016, 7, 12933 CrossRef PubMed; (i) X.-Y. Chen, K.-Q. Chen, D.-Q. Sun and S. Ye, Carbene-Catalyzed Oxidative [3 + 2] Annulation of Dioxindoles and Enals: Cross Coupling of Homoenolate and Enolate, Chem. Sci., 2017, 8, 1936 RSC; (j) X. Wu, Y. Zhang, Y. Wang, J. Ke, M. Jeret, R. N. Reddi, S. Yang, B.-A. Song and Y. R. Chi, Polyhalides as Efficient and Mild Oxidants for Oxidative Carbene Organocatalysis by Radical Processes, Angew. Chem., Int. Ed., 2017, 56, 2942 CrossRef CAS PubMed; (k) K. Zhao and D. Enders, Merging N-Heterocyclic Carbene Catalysis and Single Electron Transfer: A New Strategy for Asymmetric Transformations, Angew. Chem., Int. Ed., 2017, 56, 3754 CrossRef CAS PubMed; (l) T. Ishii, K. Ota, K. Nagao and H. Ohmiya, N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Vicinal Alkylacylation of Alkenes, J. Am. Chem. Soc., 2019, 141, 14073 CrossRef CAS PubMed; (m) T. Ishii, Y. Kakeno, K. Nagao and H. Ohmiya, N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation of Aldehydes, J. Am. Chem. Soc., 2019, 141, 3854 CrossRef CAS PubMed; (n) R. Song and Y. R. Chi, N-Heterocyclic Carbene Catalyzed Radical Coupling of Aldehydes with Redox-Active Esters, Angew. Chem., Int. Ed., 2019, 58, 8628 CrossRef CAS PubMed; (o) V. Regnier, E. A. Romero, F. Molton, R. Jazzar, G. Bertrand and D. Martin, What Are the Radical Intermediates in Oxidative N-Heterocyclic Carbene Organocatalysis?, J. Am. Chem. Soc., 2019, 141, 1109 CrossRef CAS PubMed; (p) K. Ota, K. Nagao and H. Ohmiya, N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Synthesis of δ-Ketocarbonyls, Org. Lett., 2020, 22, 3922 CrossRef CAS PubMed; (q) J.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng and Q.-. Z. Li, Radical Acylfluoroalkylation of Olefins through N-Heterocyclic Carbene Organocatalysis, Angew. Chem., 2020, 59, 1863 CrossRef CAS PubMed; (r) B. Zhang, Q. Peng, D. Guo and J. Wang, NHC-Catalyzed Radical Trifluoromethylation Enabled by Togni Reagent, Org. Lett., 2020, 22, 443 CrossRef CAS PubMed; (s) H.-B. Yang, Z.-H. Wang, J.-M. Li and C. Wu, Modular synthesis of α-aryl β-perfluoroalkyl ketones via N-heterocyclic carbene catalysis, Chem. Commun., 2020, 56, 3801 RSC; (t) I. Kim, H. Im, H. Lee and S. Hong, N-Heterocyclic Carbene-Catalyzed Deaminative Cross-Coupling of Aldehydes with Katritzky Pyridinium Salts, Chem. Sci., 2020, 11, 3192 RSC; (u) T. Ishii, K. Nagao and H. Ohmiya, Recent Advances in N-Heterocyclic Carbene-Based Radical Catalysis, Chem. Sci., 2020, 11, 5630 RSC.
- (a) D. T. Cohen and K. A. Scheidt, Cooperative Lewis acid/N-heterocyclic carbene catalysis, Chem. Sci., 2012, 3, 53 RSC; (b) M. H. Wang and K. A. Scheidt, Cooperative Catalysis and Activation with N-Heterocyclic Carbenes, Angew. Chem., Int. Ed., 2016, 55, 14912 CrossRef CAS PubMed; (c) K. Nagao and H. Ohmiya, N-Heterocyclic Carbene (NHC)/Metal Cooperative Catalysis, Top. Curr. Chem., 2019, 377, 35 CrossRef PubMed; (d) H. Ohmiya, N-Heterocyclic Carbene-Based Catalysis Enabling Cross-Coupling Reactions, ACS Catal., 2020, 10, 6862 CrossRef CAS.
- (a) Z. Chen, X. Yu and J. Wu, Silver Triflate and N-Heterocyclic Carbene Co-catalyzed Reaction of N′-(2-alkynylbenzylidene) Hydrazide, Methanol with α,β-Unsaturated Aldehyde, Chem. Commun., 2010, 46, 6356 RSC; (b) K. Liu, M. T. Hovey and K. A. Scheidt, A Cooperative N-Heterocyclic Carbene/Palladium Catalysis System, Chem. Sci., 2014, 5, 4026 RSC; (c) K. Namitharan, T. Zhu, J. Cheng, P. Zheng, X. Li, S. Yang, B.-A. Song and Y. R. Chi, Metal and Carbene Organocatalytic Relay Activation of Alkynes for Stereoselective Reactions, Nat. Commun., 2014, 5, 3982 CrossRef CAS PubMed; (d) Y. Bai, S. Xiang, M. L. Leow and X.-W. Liu, Dual-function Pd/NHC catalysis: tandem allylation–isomerization–conjugate addition that allows access to pyrroles, thiophenes and furans, Chem. Commun., 2014, 50, 6168 RSC; (e) Y. Bai, W.-L. Leng, Y. Li and X.-W. Liu, A Highly Efficient Dual Catalysis Approach for C-Glycosylation: Addition of (o-Azaaryl) Carboxaldehyde to Glycals, Chem. Commun., 2014, 50, 13391 RSC; (f) C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, Cooperative N-Heterocyclic Carbene/Palladium-Catalyzed Enantioselective Umpolung Annulations, J. Am. Chem. Soc., 2016, 138, 7840 CrossRef CAS PubMed; (g) C. Guo, D. Janssen-Müller, M. Fleige, A. Lerchen, C. G. Daniliuc and F. Glorius, Mechanistic Studies on a Cooperative NHC Organocatalysis/Palladium Catalysis System: Uncovering Significant Lessons for Mixed Chiral Pd(NHC)(PR3) Catalyst Design, J. Am. Chem. Soc., 2017, 139, 4443 CrossRef CAS PubMed; (h) S. Singha, T. Patra, C. G. Daniliuc and F. Glorius, Highly Enantioselective [5 + 2] Annulations through Cooperative N-Heterocyclic Carbene (NHC) Organocatalysis and Palladium Catalysis, J. Am. Chem. Soc., 2018, 140, 3551 CrossRef CAS; (i) S. Yasuda, T. Ishii, S. Takemoto, H. Haruki and H. Ohmiya, Synergistic N-Heterocyclic Carbene/Palladium-Catalyzed Reactions of Aldehyde Acyl Anions with either Diarylmethyl or Allylic Carbonates, Angew. Chem., Int. Ed., 2018, 57, 2938 CrossRef CAS; (j) Z.-J. Zhang, L. Zhang, R.-L. Geng, J. Song, X.-H. Chen and L.-Z. Gong, N-Heterocyclic Carbene/Copper Cooperative Catalysis for the Asymmetric Synthesis of Spirooxindoles, Angew. Chem., Int. Ed., 2019, 58, 12190 CrossRef CAS PubMed; (k) S. Takemoto, T. Ishii, S. Yasuda and H. Ohmiya, Synergistic N-Heterocyclic Carbene/Palladium-Catalyzed Allylation of Aldehydes with Allylic Carbonates, Bull. Chem. Soc. Jpn., 2019, 92, 937 CrossRef CAS; (l) H. Haruki, S. Yasuda, K. Nagao and H. Ohmiya, Dehydrative Allylation between Aldehydes and Allylic Alcohols through Synergistic N-Heterocyclic Carbene/Palladium Catalysis, Chem. – Eur. J., 2019, 25, 724 CrossRef CAS PubMed; (m) N. Ohnishi, S. Yasuda, K. Nagao and H. Ohmiya, Synergistic N-Heterocyclic Carbene/Palladium-Catalyzed Aldehyde Acylation of Allylic Amines, Asian J. Org. Chem., 2019, 8, 1133 CrossRef CAS; (n) S. Singha, E. Serrano, S. Mondal, C. G. Daniliuc and F. Glorius, Diastereodivergent Synthesis of Enantioenriched α,β-Disubstituted γ-Butyrolactones via Cooperative N-Heterocyclic Carbene and Ir Catalysis, Nat. Catal., 2020, 3, 48 CrossRef CAS; (o) W. Yang, B. Ling, B. Hu, H. Yin, J. Mao and P. J. Walsh, Synergistic N-Heterocyclic Carbene/Palladium-Catalyzed Umpolung 1, 4-Addition of Aryl Iodides to Enals, Angew. Chem., 2020, 59, 161 CrossRef CAS.
- (a) X. Zhao, D. A. DiRocco and T. Rovis, N-Heterocyclic Carbene and Brønsted Acid Cooperative Catalysis: Asymmetric Synthesis of trans-γ-Lactams, J. Am. Chem. Soc., 2011, 133, 12466 CrossRef CAS; (b) Z. Jin, J. Xu, S. Yang, B.-A. Song and Y. R. Chi, Enantioselective Sulfonation of Enones with Sulfonyl Imines by Cooperative N-Heterocyclic-Carbene/Thiourea/Tertiary-Amine Multicatalysis, Angew. Chem., Int. Ed., 2013, 52, 12354 CrossRef CAS PubMed; (c) Y.-F. Tong, J.-H. Mao, S. Wu, Y. Zhao and Y. Cheng, Changing the Reaction Pathway by NHC/Brønsted Base Cooperative Catalysis: Highly Stereoselective Synthesis of Multifunctional Benzo[a]fluoren-11-ones from the Dimerization of 2-(Aroylvinyl)arylaldehydes, J. Org. Chem., 2014, 79, 2075 CrossRef CAS; (d) S. W. Youn, H. S. Song and J. H. Park, Asymmetric Domino Multicatalysis for the Synthesis of 3-Substituted Phthalides: Cinchonine/NHC Cooperative System, Org. Lett., 2014, 16, 1028 CrossRef CAS; (e) J. Chen and Y. Huang, Asymmetric Catalysis with N-Heterocyclic Carbenes as Non-covalent Chiral Templates, Nat. Commun., 2014, 5, 3437 CrossRef PubMed; (f) J. L. Li, B. Sahoo, C. G. Daniliuc and F. Glorius, Conjugate Umpolung of β,β-Disubstituted Enals by Dual Catalysis with an N-Heterocyclic Carbene and a Brønsted Acid: Facile Construction of Contiguous Quaternary Stereocenters, Angew. Chem., Int. Ed., 2014, 53, 10515 CrossRef CAS PubMed; (g) S. Bera, R. C. Samanta, C. G. Daniliuc and A. Studer, Asymmetric Synthesis of Highly Substituted β-Lactones through Oxidative Carbene Catalysis with LiCl as Cooperative Lewis Acid, Angew. Chem., Int. Ed., 2014, 53, 9622 CrossRef CAS PubMed; (h) Z.-Q. Liang, D.-L. Wang, H.-M. Zhang and S. Ye, Enantioselective synthesis of bicyclic δ-lactones via N-heterocyclic Carbene-Catalyzed Cascade Reaction, Org. Lett., 2015, 17, 5140 CrossRef CAS PubMed; (i) Z. Wu, F. Li and J. Wang, Intermolecular Dynamic Kinetic Resolution Cooperatively Catalyzed by an N-Heterocyclic Carbene and a Lewis Acid, Angew. Chem., Int. Ed., 2015, 54, 1629 CrossRef CAS; (j) J. Xu, X. Chen, M. Wang, P. Zheng, B.-A. Song and Y. R. Chi, Aminomethylation of Enals through Carbene and Acid Cooperative Catalysis: Concise Access to β2-Amino Acids, Angew. Chem., Int. Ed., 2015, 54, 5161 CrossRef CAS PubMed; (k) M. H. Wang, D. T. Cohen, C. B. Schwamb, R. K. Mishra and K. A. Scheidt, Enantioselective β-Protonation by a Cooperative Catalysis Strategy, J. Am. Chem. Soc., 2015, 137, 5891 CrossRef CAS.
- D. A. DiRocco and T. Rovis, Catalytic Asymmetric α-Acylation of Tertiary Amines Mediated by a Dual Catalysis Mode: N-Heterocyclic Carbene and Photoredox Catalysis, J. Am. Chem. Soc., 2012, 134, 8094 CrossRef CAS PubMed.
- L. Dai, Z.-H. Xia, Y.-Y. Gao, Z.-H. Gao and S. Ye, Visible-Light-Driven N-Heterocyclic Carbene-catalyzed γ- and ε-Alkylation with Alky, Angew. Chem., Int. Ed., 2019, 58, 18124 CrossRef CAS PubMed.
- L. Dai and S. Ye, Photo/N-Heterocyclic Carbene Co-catalyzed Ring Opening and γ-Alkylation of Cyclopropane Enal, Org. Lett., 2020, 22, 986 CrossRef CAS PubMed.
- S. B. Poh, J.-Y. Ong, S. Lu and Y. Zhao, Highly Regio- and Stereodivergent Access to 1, 2-Amino Alcohols or 1, 4-Fluoro Alcohols by NHC-Catalyzed Ring Opening of Epoxy enals, Angew. Chem., Int. Ed., 2018, 57, 1645 CrossRef CAS PubMed.
- Z.-H. Xia, L. Dai, Z.-H. Gao and S. Ye, N-Heterocyclic Carbene/Photo-Cocatalyzed Oxidative Smiles Rearrangement: Synthesis of Aryl Salicylates from O-Aryl Salicylaldehydes, Chem. Commun., 2020, 56, 1525 RSC.
- Z.-H. Gao, Z.-H. Xia, L. Dai and S. Ye, N-Heterocyclic Carbene Catalyzed Photooxidation: Intramolecular Cross Dehydrogenative Coupling of Tetrahydroisoquinoline-Tethered Aldehydes, Adv. Synth. Catal., 2020, 362, 1819 CrossRef CAS.
- A. Mavroskoufis, K. Rajes, P. Golz, A. Agrawal, V. Ruß, J. P. Götze and M. N. Hopkinson, N-Heterocyclic Carbene-Catalyzed Photoenolization Diels-Alder Reaction of Acid Fluorides, Angew. Chem., 2020, 59, 3190 CrossRef CAS PubMed.
- A. V. Davies, K. P. Fitzpatrick, R. C. Betori and K. A. Scheidt, Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids, Angew. Chem., 2020, 59, 9143 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2020 |