Recent advances in cyclization reactions of unsaturated oxime esters (ethers): synthesis of versatile functionalized nitrogen-containing scaffolds
Chen
Chen
 *a,
Jinghui
Zhao
a,
Xiaonan
Shi
a,
Liying
Liu
a,
Yan-Ping
Zhu
b,
Wan
Sun
a and
Bolin
Zhu
*a,
Jinghui
Zhao
a,
Xiaonan
Shi
a,
Liying
Liu
a,
Yan-Ping
Zhu
b,
Wan
Sun
a and
Bolin
Zhu
 *a
*a
aTianjin Key Laboratory of Structure and Performance for Functional Molecules, College of Chemistry, Tianjin Normal University, Tianjin 300387, P. R. China. E-mail: hxxycc@tjnu.edu.cn; hxxyzbl@tjnu.edu.cn
bSchool of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Collaborative Innovation Center of Advanced Drug Delivery System and Biotech Drugs in Universities of Shandong, Yantai University, Shandong, Yantai 264005, P. R. China
First published on 5th June 2020
Abstract
Cyclization/functionalization of unsaturated oxime esters (ethers) is a powerful strategy for the synthesis of versatile functionalized nitrogen-containing scaffolds, which are valuable structural motifs in numerous bioactive molecules. This review provides an overview of the recent advances in cascade cyclization of unsaturated oxime esters (ethers) with different functional group reagents. Typical examples are listed and mechanistic aspects are discussed in detail. Moreover, the remaining challenges and future perspectives are presented at the end.
1. Introduction
Nitrogen-containing molecules have gained great attention over the past few decades, as they constitute the core structure of drugs, materials and agrochemicals.1,2 In particular, they hold a dominant position in drug discovery. It is known that approximately eighty percent of the top 20 best-selling brand name drugs incorporate nitrogen atoms, in which two-thirds of them contain N-heterocycles.3 Among them, functionalized pyrrolines and related scaffolds are valuable structural motifs in numerous bioactive molecules, pharmaceuticals and alkaloid natural products.4–6 Therefore, the development of effective synthetic methodologies for rapidly constructing various functionalized pyrrolines and related scaffolds from simple and readily available starting materials has been of great interest to many organic chemists.Over the past few decades, oxime esters (ethers) have been one of the most widely used starting materials for the synthesis of small molecule nitrogen-containing heterocycles,7–31 which can be easily prepared from the corresponding ketones. This is mainly due to the viable and facile oxidative addition of the N–O bond to low valent transition metal centers. In addition, an iminyl radical could be generated via N–O bond homolytic cleavage. In particular for γ,δ-unsaturated oxime esters (ethers), the N–O bond cleavage is followed by an intramolecular imino-functionalization process, which delivers diverse functionalized nitrogen-containing scaffolds. Recently, great progress has been made in this field. In this review, we mainly focus on the recent advancements in the study of the synthesis of versatile functionalized nitrogen-containing scaffolds from unsaturated oxime esters (ethers). We hope that this review would serve as a reference for organic chemists who are interested in developing novel synthetic methodologies using unsaturated oxime esters (ethers).
2. Cyclization reactions of γ,δ-unsaturated oxime esters (ethers)
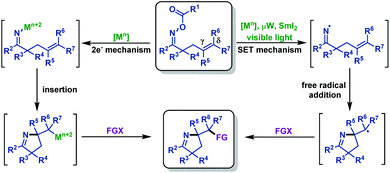
γ,δ-Unsaturated oxime esters (ethers) are important starting materials and useful building blocks in organic synthesis, which can undergo N–O bond cleavage followed by an intramolecular imino-functionalization process, delivering diverse functionalized pyrroline moieties. Recently, much progress has been achieved in this field. In the section, we mainly focus on the recent advances in the synthesis of diverse functionalized pyrrolines and related scaffolds from γ,δ-unsaturated oxime esters. These reactions are classified by the type of N–O bond cleavage, including N–O bond homolytic cleavage and N–O bond oxidative addition (Scheme 1).2.1 Cyclization reactions involving iminyl radical intermediates
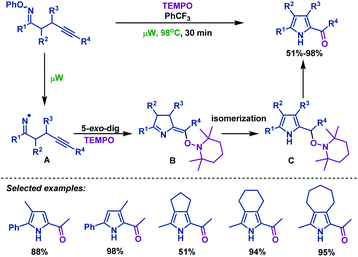
Iminyl radical cyclizations have emerged as an important tool that allows the efficient construction of nitrogen-containing heterocycles. During the past few decades, several important advancements in iminyl radical cyclizations of γ,δ-unsaturated oxime esters (ethers) have been made by organic chemists. Iminyl radicals are useful intermediates that can be generated from the N–O bond homolytic cleavage of γ,δ-unsaturated oxime esters (ethers). Typical conditions for generating iminyl radicals include microwave heating,32–36 transition metal catalysis,37–54 light irradiation55–62 and SmI2 irradiation.63In 2015, Castle et al.36 reported a microwave-promoted iminyl radical cyclization of γ,δ-unsaturated oxime ethers with TEMPO, which afforded a variety of 2-acylpyrroles in moderate to good yields (Scheme 3). PhCF3 was used as a solvent to effectively facilitate radical cyclization and avoid hydrogen atom abstraction. Mechanistically, N–O bond homolytic cleavage of γ,δ-unsaturated oxime ethers was induced by microwave heating to afford iminyl radical intermediate A. Then A undergoes an intramolecular 5-exo-dig radical cyclization to generate a vinyl radical intermediate, which could be captured by TEMPO to afford B. Subsequently, isomerization of B delivers the more stable pyrrole intermediate C, which undergoes H abstraction to furnish the desired 2-acylpyrroles.
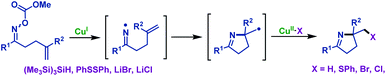
2.1.2.1 Copper-catalyzed/promoted reactions. Copper-catalyzed/promoted cascade radical cyclization/functionalization reactions of γ,δ-unsaturated oxime esters are the most effective and practical synthetic strategies to synthesize versatile functionalized pyrrolines. For instance, Narasaka and co-workers37 developed a novel copper-catalyzed imino-functionalization of γ,δ-unsaturated oxime esters with different radical trapping reagents, such as (Me3Si)3SiH, PhSSPh, LiBr and LiCl (Scheme 4). By using this protocol, a variety of functionalized pyrrolines are thus prepared catalytically, whereas the formation of tetrahydropyridine is hard to proceed from δ,ε-unsaturated oxime esters.
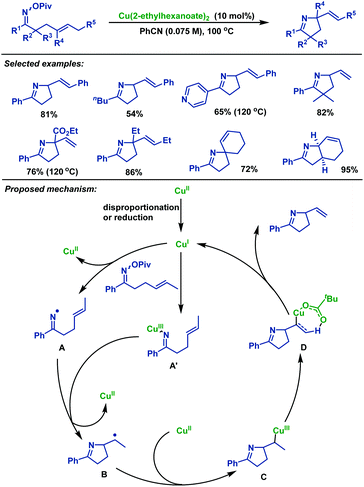
Later, Bower and colleagues38 described nice copper-catalyzed Heck-like cyclizations of γ,δ-unsaturated oxime esters in 2014, furnishing a series of structurally diverse alkene-containing pyrrolines in moderate to good yields (Scheme 5). Notably, this protocol tolerates less activated O-pivaloyl oxime. This facet is in stark contrast to the work with Pd-systems,39–41 where O-pentafluorobenzoyl oximes are necessary for efficient cyclization. The reaction mechanism may involve the following steps: firstly, CuI species was formed in situ via disproportionation or reduction of Cu(2-ethylhexanoate)2. Next, pathways proceed via either the generation of imino-CuIII intermediate A′ or iminyl radical intermediate A; in the former case cyclization occurs by the homolytic cleavage of the N–Cu bond. Subsequently, alkyl radical intermediate B is trapped by CuII species to provide alkyl-CuIII intermediate C, followed by syn β-H elimination to generate alkene-containing pyrrolines.
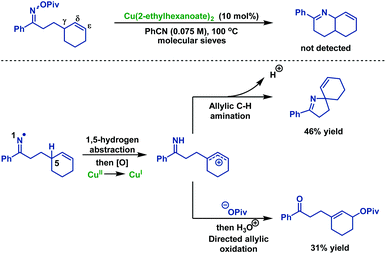
The author then explored the possibility of 6-ring cyclization of δ,ε-unsaturated oxime esters (Scheme 6). Surprisingly, exposure of δ,ε-unsaturated oxime esters under optimal conditions did not afford the Heck-type product. Instead, the allylic C–H amination or oxidation products were achieved in 46% and 31% yields, respectively.
In 2018, the group of Zhu42 realized a copper-catalyzed domino cyclization/trifluoromethylthiolation of γ,δ-unsaturated oxime esters for the synthesis of a variety of SCF3-containing pyrrolines (Scheme 7). A series of γ,δ-unsaturated oxime esters were investigated, leading to SCF3-substituted pyrrolines in good yields. The reaction enables the simultaneous formation of a C(sp3)–SCF3 bond and a C–N bond, and is amenable for gram-scale synthesis. Mechanistically, the N–O bond of γ,δ-unsaturated oxime esters was cleaved by CuSCF3, which was generated from the metathesis between Cu(I) and AgSCF3. Consequently, iminyl radical intermediate A and Cu(II)SCF3 species were formed. Next, A underwent 5-exo-trig cyclization to afford alkyl radical intermediate B, which could be captured by Cu(II)SCF3, followed by C–Cu(III)–SCF3 reductive elimination to construct a C–SCF3 bond. Alternatively, B could be oxidized to carbocation D by Cu(II)SCF3, which can react with the SCF3 anion to give the SCF3-substituted pyrrolines (Scheme 7).
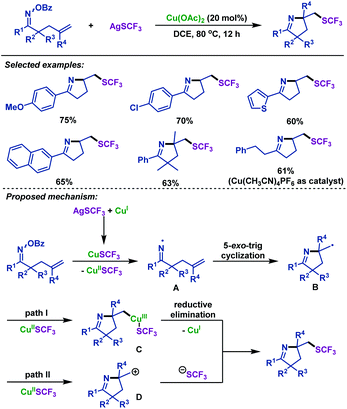 | ||
| Scheme 7 Zhu's copper-catalyzed imino-trifluoromethylthiolation of γ,δ-unsaturated oxime esters with AgSCF3. | ||
In 2019, our group43 reported a highly efficient approach for the cascade radical cyclization/sulfonylation of γ,δ-unsaturated oxime esters with sodium sulfinates (Scheme 8). Both sodium arylsulfinates and sodium alkylsulfinates were compatible in this reaction, and the desired imino-sulfonylation products were obtained in moderate to excellent yields. Notably, sodium heteroarylsulfinate was also tolerated in this transformation. Based on the control experimental results and previous reports, we proposed a plausible mechanism. First, sodium sulfinate was oxidized by Cu(II) to give a sulfonyl radical and Cu(I) species. Then, an iminyl radical intermediate was formed through N–O bond homolytic cleavage with the assistance of Cu(I). Next, it underwent an intramolecular 5-exo-trig radical cyclization to form carbon radical intermediate B, which was trapped by the sulfonyl radical to afford the sulfonyl-containing pyrrolines.
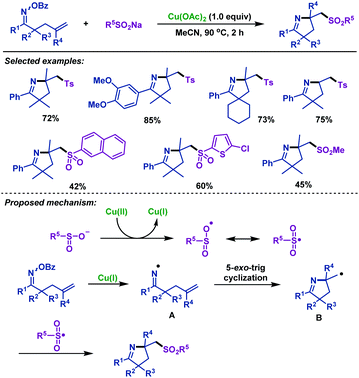 | ||
| Scheme 8 Zhu and Chen's copper-promoted imino-sulfonylation of γ,δ-unsaturated oxime esters with sodium sulfinates. | ||
In addition, we also reported CuX-promoted (X = CN, SCN) imino-thiocyanation and imino-cyanogenation of γ,δ-unsaturated oxime esters to furnish a series of cyano- and thiocyano-containing pyrrolines in good yields (Scheme 9).43 The limitation of this transformation was that γ,δ-unsaturated oxime ester without geminal disubstitution failed to undergo the cyclization reaction. This was probably due to the lack of the Thorpe–Ingold effect, which aided gem-dimethyl substrates, thus favoring radical cyclization. Notably, a series of synthetic transformations were carried out, which illustrated the synthetic potential of our prepared cyano- and thiocyano-functionalized pyrrolines.
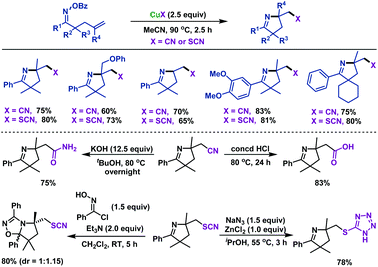 | ||
| Scheme 9 Zhu and Chen's CuX (X = CN or SCN) promoted imino-cyanogenation and imino-thiocyanation of γ,δ-unsaturated oxime esters. | ||
Soon afterwards, our group44 reported a copper-catalyzed imino-halogenation of γ,δ-unsaturated oxime esters with KX (Scheme 10). A variety of structurally diversiform 2-halomethyl pyrrolines were efficiently synthesized by using this protocol. We proposed that a Cu(I)-initiated iminyl radical cyclization process was involved in this transformation.
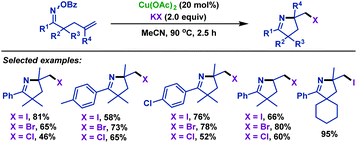 | ||
| Scheme 10 Zhu and Chen's copper-catalyzed imino-halogenation of γ,δ-unsaturated oxime esters with KX. | ||
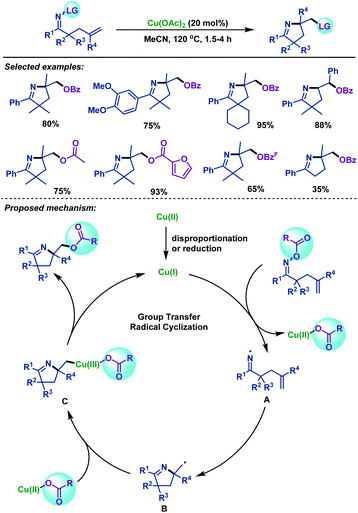
Although an array of methodologies are available for imino-functionalization of γ,δ-unsaturated oxime esters for the synthesis of versatile functionalized pyrrolines, the leaving group was not effectively utilized in the imino-functionalization process, leading to reduced atom economy. Therefore, the utilization of the leaving group as the coupling partners in the imino-functionalization of γ,δ-unsaturated oxime esters needs to be investigated. In 2019, our group45 realized an atom-economical copper-catalyzed group-transfer radical cyclization (GTRC) of γ,δ-unsaturated oxime esters, during which a five-membered ring, a new C–O bond and a new C–N bond were formed in a one-step manner. This reaction effectively utilizes the leaving group as the ArC(O)O source for the first time, avoiding the use of additional reagents. A variety of ester-containing pyrrolines were obtained under oxidant-free and ligand-free conditions in good yields (Scheme 11). The possible mechanism for this copper-catalyzed GTRC reaction was proposed. Initially, a CuI species was formed in situ via the reduction or disproportionation of Cu(OAc)2. Next, N–O bond homolytic cleavage led to the formation of iminyl radical intermediate A, which underwent 5-exo-trig radical cyclization to form alkyl radical intermediate B. Finally, intermediate B is trapped by CuIIOCOR to produce CuIII intermediate C, followed by reductive elimination to provide ester-functionalized pyrrolines and CuI species.
In the same year, Wang and colleagues46 illustrated a redox-neutral copper-catalyzed diamination of γ,δ-unsaturated oxime esters with various unprotected amines. This reaction offers an efficient entry to various amine-containing pyrrolines under ligand- and oxidant-free conditions, and employs readily available unprotected amines as the nucleophilic nitrogen source. The radical clock experiment was conducted and the radical pathway for the cyclization step was proposed (Scheme 12).
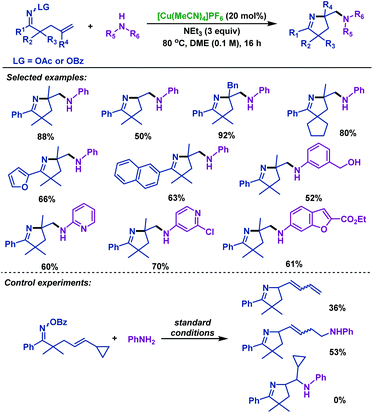 | ||
| Scheme 12 Wang's copper-catalyzed diamination of γ,δ-unsaturated oxime esters with unprotected amines. | ||
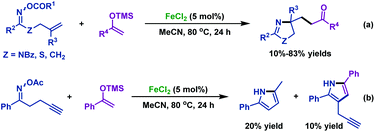
2.1.2.2 Iron-catalyzed reactions. Except for the copper catalyst mentioned above, the environmentally friendly and cheap iron catalyst can also realize the imino-functionalization of γ,δ-unsaturated oxime esters. In 2017, the Selander group47 described an iron-catalyzed coupling reaction of γ,δ-unsaturated oxime esters with silyl enol ethers (Scheme 13a). This protocol provides access to various functionalized pyrrolines via alkyl radicals, which are generated from an initially formed iminyl radical by N–O bond homolytic cleavage. It is worth noting that when alkyne-tethered oxime ester was used under optimal conditions, a mixture of products was obtained which demonstrated that the competitive pathways of the intermolecular 1,3-hydrogen transfer and the 5-exo-dig cyclization were involved (Scheme 13b).
Later, Okamoto and Ohe48 reported an iron-catalyzed imino-arylation of γ,δ-unsaturated oxime esters with various arenes (Scheme 14). Not only electron-poor and electron-rich arenes, but also heteroarenes were well tolerated in this reaction. Moreover, γ,δ-unsaturated oxime esters bearing sterically hindered alkene moieties were applicable to this reaction system. A plausible mechanism involving an iminyl radical and alkyl radical species was proposed based on radical trapping experiments. Furthermore, no kinetic isotope effect was observed in C–H bond cleavage, which demonstrated that the general homolytic aromatic substitution process might be involved.
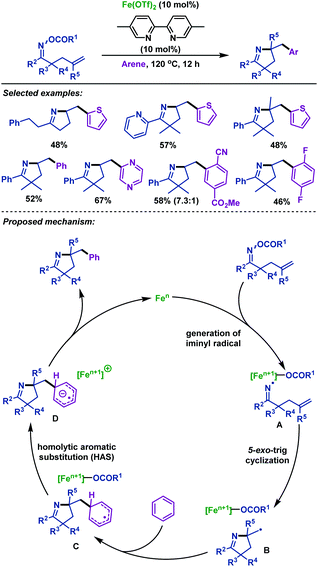 | ||
| Scheme 14 Okamoto and Ohe's iron-catalyzed imino-arylation of γ,δ-unsaturated oxime esters with arenes. | ||
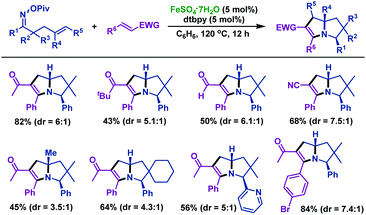
In 2018, the same group49 realized an iron-catalyzed cycloaddition reaction of γ,δ-unsaturated oxime esters with 1,2-disubstituted alkenes to synthesize diversiform tetrahydropyrrolizines, which are frequently found in bicyclic alkaloids (Scheme 15). A series of γ,δ-unsaturated oxime esters reacted with various 1,2-disubstituted alkenes smoothly, producing tetrahydropyrrolizines in moderate to satisfactory yields. Good functional group compatibility was observed in this transformation.
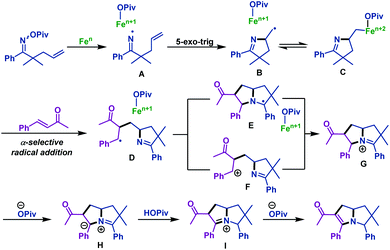
The possible mechanism of this transformation is illustrated in Scheme 16. First, N–O bond homolytic cleavage of γ,δ-unsaturated oxime esters forms iminyl radical intermediate A, which undergoes 5-exo-trig cyclization to give C-centered radical intermediate B. Normally, B may be in equilibrium with the corresponding iron adduct C. Next, α-selective radical addition with activated alkenes gives radical intermediate D, which is demonstrated by DFT analyses. Subsequently, radical cyclization of intermediate D with the imine group gives intermediate E, which is followed by oxidation to give bicyclic iminium G. Alternatively, D could also undergo the single electron oxidation by iron to form cation F, which is followed by ionic cyclization to G. Finally, deprotonation by pivalate gives azomethine ylide H, followed by deprotonation and protonation to complete the reaction.
2.1.2.3 Nickel-catalyzed/promoted reactions. Nickel is a powerful catalyst that enables the efficient construction of functionalized pyrrolines from γ,δ-unsaturated oxime esters. Since the pioneering work of the Zard group50 in 1999, further exploration by Selander's group51 and Wang's group52 has enriched and expanded the scope of this method. In 2017, Selander et al.51 reported a nickel-catalyzed imino-arylation of γ,δ-unsaturated oxime esters with boronic acids. Various arylated pyrroline derivatives were synthesized in moderate to good yields via the successive formation of C(sp3)–C(sp2) and C(sp3)–N bonds. Moreover, by using this protocol, arylated imidazoline derivatives can also be synthesized from amidooxime (Scheme 17a). It is noteworthy that a potent tubulin inhibitor, ABI-274, was obtained via three-step synthesis, as described in Scheme 17b. This strategy offers an alternative route to synthesize ABI analogues.
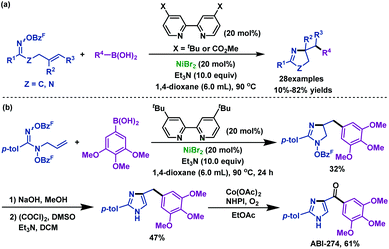 | ||
| Scheme 17 Selander's nickel-catalyzed imino-arylation of γ,δ-unsaturated oxime esters with boronic acids. | ||
2.1.2.4 Palladium-catalyzed reactions. In 2016, the Bower group53 illustrated that palladium-catalyzed cyclizations of γ,δ-unsaturated oxime esters are subject to an unusual ligand controlled mechanistic divergence (Scheme 18). Pd-Systems with electron-rich phosphines (such as P(tBu)3 and dt-bpf) promote SET-type oxidative addition. In these cases, C–N bond formation occurs via cyclization of an iminyl radical with pendant alkenes. Conversely, for electron-deficient phosphines (such as P(3,5-(CF3)2C6H3)3), N–O oxidative addition proceeds via a two-electron pathway to generate imino-palladium intermediates, which employ pendant alkenes in a Heck-type cyclization. They also demonstrated a palladium-catalyzed imino-hydrogenation of γ,δ-unsaturated oxime esters with γ-terpinene, during which the electron-rich ligand, dt-bpf, was crucial to promote SET-type oxidative addition. Furthermore, a series of mechanistic experiments were conducted to verify the SET pathway and the scope of imino-hydrogenation was outlined.
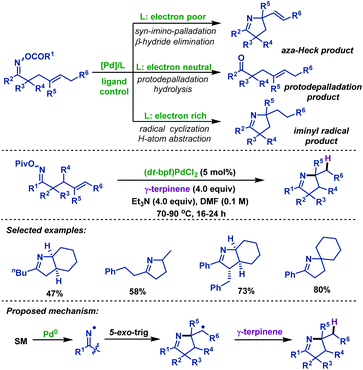 | ||
| Scheme 18 Bower's palladium-catalyzed imino-hydrogenation of γ,δ-unsaturated oxime esters with γ-terpinene. | ||
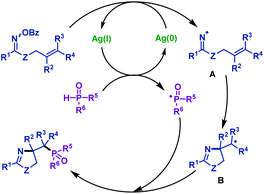
2.1.2.5 Silver promoted reactions. Recently, our group54 reported the first silver-promoted radical cyclization of γ,δ-unsaturated oxime esters with P(O)H compounds (Scheme 19). Both secondary phosphine oxides and H-phosphinates are compatible with this reaction. γ,δ-Unsaturated oxime esters with both electron-deficient and electron-rich groups could give the corresponding phosphorylated pyrrolines in good to satisfactory yields. Moreover, the heterocycle moiety was compatible with this reaction. Notably, this protocol was also efficient for trisubstituted alkenes. In addition, a phosphorylated imidazoline derivative was obtained from amidooxime under the same conditions.
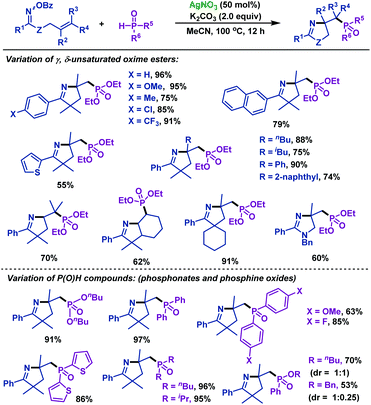 | ||
| Scheme 19 Zhu and Chen's silver-promoted imino-phosphorylation of γ,δ-unsaturated oxime esters with P(O)H compounds. | ||
Based on control experiments, a possible reaction mechanism was proposed (Scheme 20). Initially, iminyl radical intermediate A and a phosphinoyl radical were formed with the assistance of AgNO3. Subsequently, the iminyl radical intermediate A underwent an intramolecular 5-exo-trig cyclization to form a carbon radical intermediate B, followed by radical coupling with the phosphinoyl radical to afford the desired phosphorylated pyrrolines.
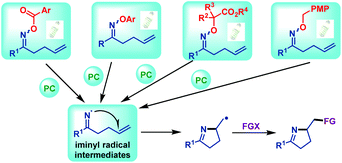 | ||
| Scheme 21 Iminyl radical generations from γ,δ-unsaturated oxime esters (ethers) via visible light irradiation. | ||
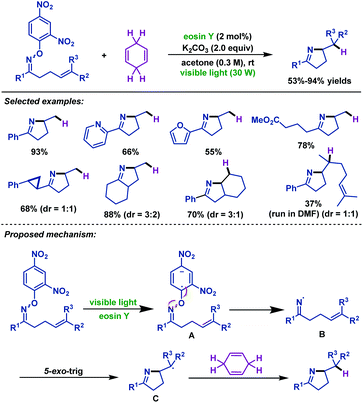
In 2015, Leonori and colleagues55 devised two complementary visible-light-mediated hydroimination and iminohydroxylation of γ,δ-unsaturated oxime ethers (Schemes 22 and 23). On account of the low reduction potentials of O-(2,4-dinitrophenyl)oximes, the cheap and easily available eosin Y could be used as the photocatalyst for hydroimination reaction. A wide range of γ,δ-unsaturated oxime ethers possessing diverse steric and electronic properties participated efficiently in this transformation. Moreover, both internal alkenes and terminal alkenes were suitable substrates for this reaction. Presumably, the reaction was initiated by visible-light promoted single-electron transfer of γ,δ-unsaturated oxime ethers to give radical anion intermediate A, followed by fragmentation to form iminyl radical intermediate B, which then underwent 5-exo-trig cyclization to give alkyl radical intermediate C. Finally, C abstracts H from CHD to give the desired hydroimination product (Scheme 22).
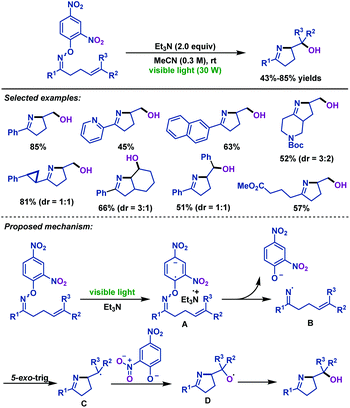
Meanwhile, the reaction conditions for visible-light-mediated iminohydroxylation of γ,δ-unsaturated oxime ethers were identified by the Leonori group (Scheme 23).55 Utilizing the visible-light-mediated triethylamine triggered iminohydroxylation process, various iminoalcohols were synthesized. It is worth mentioning that the dinitrophenyl of oxime ether plays two roles in the reaction. Aside from the role of reducing the bond energy of the N–O bond to facilitate homolytic scission, it can also afford the oxygen atom to the final product. A mechanism involving visible-light-mediated SET from triethylamine to the substrate and the oxygen atom of the product originated from the nitro groups of the aromatic unit was proposed by the authors.
Since 2016, two research groups56,57 have reported the visible light-promoted imino-functionalization of γ,δ-unsaturated oxime esters with silyl enol ethers independently. First, Feng and Loh56 realized the synthesis of pyrroline derivatives using fac-[Ir-(ppy)3] as the photoredox catalyst (Scheme 24a). The group of Wu57 expanded the scope of this reaction, and they achieved the imino-sulfonylation of γ,δ-unsaturated oxime esters with silyl enol ethers and DABSO. This tandem radical process involved iminyl radical-mediated cyclization and insertion of sulfur dioxide, which was trapped by silyl enol ethers, and afforded a variety of sulfonated pyrrolines (Scheme 24b).
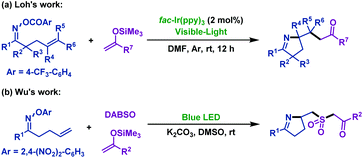 | ||
| Scheme 24 Visible light-promoted imino-functionalization of γ,δ-unsaturated oxime esters (ethers) with silyl enol ethers. | ||
In 2017, Studer et al.58 described the visible-light-promoted carboimination of γ,δ-unsaturated oxime ethers with Michael acceptors to afford pyrroline derivatives in good yields. A wide range of Michael acceptors, such as phenyl vinyl ketone, methyl vinyl ketone, α,β-unsaturated amides and phosphonates, are compatible with this reaction. Furthermore, the indolizidine skeleton could be easily synthesized from a prepared pyrroline derivative by this method. More remarkably, they applied α-imino-oxy propionic acids as a new type of efficient precursor to generate iminyl radicals upon SET oxidation (Scheme 25).
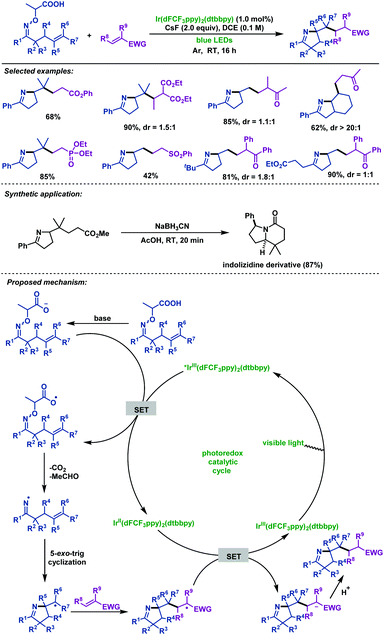 | ||
| Scheme 25 Studer's visible-light-promoted carboimination of γ,δ-unsaturated oxime ethers with Michael acceptors. | ||
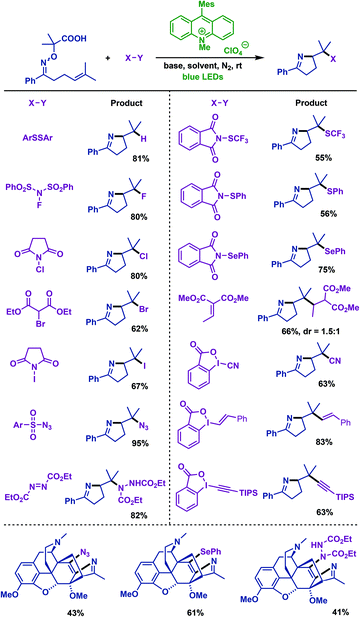
In the same year, Leonori and co-workers59 described visible-light-mediated decarboxylation radical cascade cyclization of γ,δ-unsaturated oxime ethers with a variety of SOMOphiles, which include ArSSAr, NCS, NIS, diethyl-bromo-malonate, NFSI, DEAD, ArSO2N3, Michael acceptor, IBX reagents etc. A variety of polyfunctionalized pyrrolines were obtained in favourable yields. The addition of two methyl groups at the methylenic position of oxime ethers unit could increase the electron density of carboxylate and decrease its Eox1/2 (1.65 V). Notably, by using this protocol, a molecule with a complex structure, such as the functionalized morphane derivative thevinone, was successfully involved in the formation of functionalized pyrroline products via imino-amination, imino-azidation and imino-selenation of γ,δ-unsaturated oxime ethers (Scheme 26).
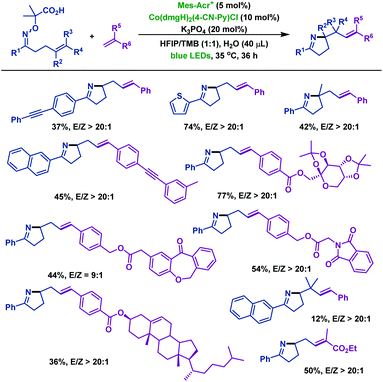
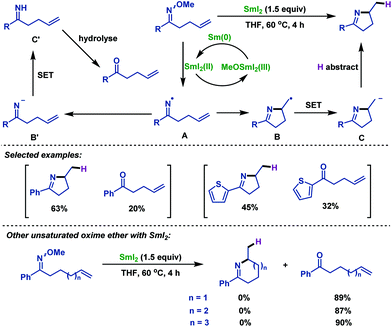
Recently, Liu and co-workers60 reported a novel radical cyclization of γ,δ-unsaturated oxime ethers via synergistic photoredox organocatalysis and cobalt catalysis. Diverse γ,δ-unsaturated oxime ethers, bearing different substituents, smoothly underwent radical cyclization reactions, and delivered a range of alkene-containing pyrrolines. The reaction was initiated by oxidative decarboxylation of oxime ethers, generating iminyl radical intermediate A with loss of acetone and CO2. Next, intermediate A underwent 5-exo-trig cyclization to deliver alkyl radical intermediate B. Subsequently, B reacted with Co(II) species to form Co(III) intermediate C, which is followed by β-H elimination to furnish alkene-containing pyrrolines (Scheme 27).
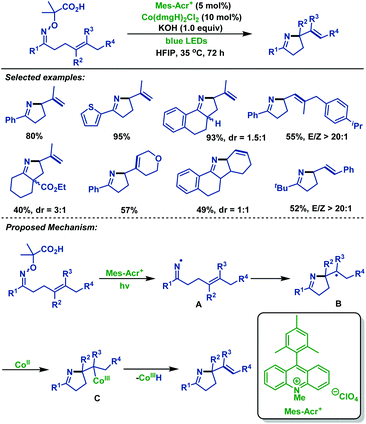 | ||
| Scheme 27 Liu's radical cyclization of γ,δ-unsaturated oxime ethers via synergistic photoredox organocatalysis and cobalt catalysis. | ||
In addition, the Liu group60 investigated the cascade radical cyclization of γ,δ-unsaturated oxime ethers with terminal alkenes (Scheme 28). A series of E-selective alkene-functionalized pyrrolines were obtained in acceptable yields. Notably, the site-selective β-H elimination process of Co(III) intermediates led to the thermodynamically stable internal alkenes as products, and the terminal alkene products were not observed in the reaction.
In the abovementioned methods, iminyl radicals were formed via the N–O bond cleavage of γ,δ-unsaturated oxime ethers under photoredox conditions. However, the re-oxidation reagents were required to regenerate the photocatalyst in most of the cases. Itoh et al.61 developed an economical and novel iminyl radical evolution method using O-PMB oxime ether in 2018. They realized visible-light-mediated hydroimination of γ,δ-unsaturated oxime ethers with 2-butanone. No additional reagent was used in this transformation because of the hydrogen atom being circulated between 2-butanone and 1-Cl-AQN, which was abstracted from the benzylic position of γ,δ-unsaturated oxime ethers. The author assumed the mechanism as follows: first, benzyl radical intermediate A was formed via hydrogen atom abstraction of excited 1-Cl-AQN from benzyl oxime ether. Then, A immediately underwent β-scission to produce iminyl radical intermediate B and p-anisaldehyde. Subsequently, formation of intermediate C was noted via 5-exo-trig cyclization of B. Next, intermediate C trapped the hydrogen atom from 2-butanone, and delivered the final pyrroline derivatives. 2-Butanone was regenerated by the hydrogen abstraction from AQH˙, thus regenerating AQN to complete the catalytic cycle (Scheme 29).
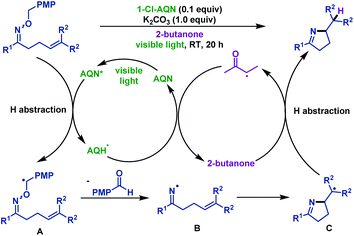 | ||
| Scheme 29 Itoh's visible-light-mediated hydroimination of γ,δ-unsaturated oxime ethers with 2-butanone. | ||
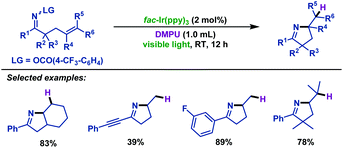
In addition, Loh and co-workers62 realized the visible-light-promoted hydroimination of γ,δ-unsaturated oxime ethers with DMPU (N,N’-dimethylpropylene urea) to synthesize pyrroline derivatives (Scheme 30). Different substrates exhibited an outstanding performance, producing the desired products in moderate to good yields. It is noteworthy that DMPU revealed triple roles in this transformation, including catalyst regeneration reagent, H-donor for the formation of products and solvent.
2.2. Cyclization reactions of γ,δ-unsaturated oxime esters via N–O bond oxidative addition
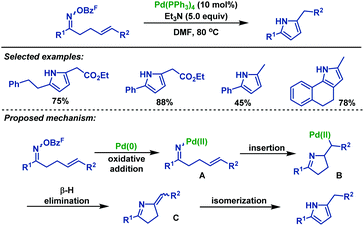 | ||
| Scheme 32 Narasaka's palladium-catalyzed Heck-type cyclization of γ,δ-unsaturated oxime: synthesis of pyrrole derivatives. | ||
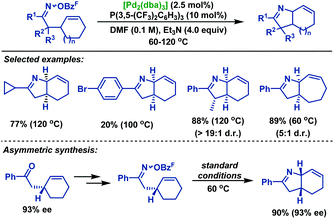
In 2012, Bower and co-workers39 reported a palladium-catalyzed Heck type cyclization of γ,δ-unsaturated oxime esters with cyclic olefins (Scheme 33). This transformation is reliant upon the use of electron-deficient phosphine ligands P(3,5-(CF3)2C6H3)3, probably due to three factors: (1) it makes the Pd center more electron-deficient and increases the stability of the putative imino-PdII intermediate; (2) the imine moiety gives greater σ donation and makes the imino-Pd bond stronger, which reduces protodepalladation and avoids the formation of ketone hydrolysis byproducts; and (3) the electron-deficient imino-PdII intermediate may accelerate the rate of the iminopalladation process. A variety of perhydroindoles and related scaffolds were obtained in moderate yields by using P(3,5-(CF3)2C6H3)3 as the ligand. However, the corresponding products were not detected from aldoxime ester substrates probably because the Beckmann rearrangement predominated to deliver the corresponding nitriles, which were observed as byproducts. It should be stressed that this methodology is readily translated to asymmetric synthesis. When the enantioenriched substrate (93% ee) was subjected to the standard conditions, no loss of the enantiomeric excesses was observed in the products.
In 2013, the Bower group40 achieved an efficient palladium-catalyzed cyclization of oxime esters with 1,1-disubstituted alkenes to synthesize a range of α,α-disubstituted pyrrolines in moderate to good yields (Scheme 34). For C–N bond formation to occur, the N–PdII bond of the imino-PdII intermediate must be oriented towards the alkene. Thus, substrates bearing smaller R1 groups show lower efficiency, probably because the steric factor is less effective at enforcing this configuration. Moreover, they also demonstrated the catalytic asymmetric variants of this chemistry. By using TADDOL-derived phosphoramidite as the chiral ligand, the desired pyrrolines were effectively generated in moderate yields, albeit with low ee values. More importantly, the TADDOL scaffold could be readily modified, which gives an opportunity to tune steric and electronic properties to find a more suitable chiral ligand.
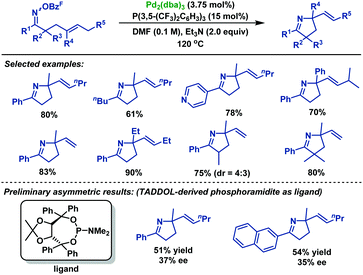 | ||
| Scheme 34 Bower's palladium-catalyzed Narasaka–Heck cyclizations of oxime esters with 1,1-disubstituted alkenes. | ||
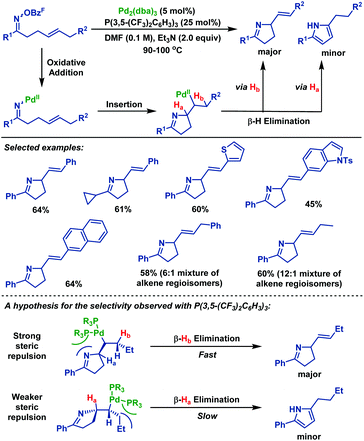
In the same year, Bower et al.41 demonstrated a substrate and catalyst-controlled strategy for selective pyrroline formation (vs. pyrrole) of oxime esters with 1,2-dialkylated alkenes (Scheme 35). Notably, in the cases where C–Hb is not benzylic, the corresponding pyrrolidine products were obtained with good selectivity over the undesired pyrroles. The authors proposed that selective β-hydride elimination could be facilitated by the interplay of the sterically demanding ligand system. The electronic and steric properties of P(3,5-(CF3)2C6H3)3 were crucial for both efficient cyclization and selective pyrroline formation. By using this protocol, a wide range of pyrrolidine derivatives were obtained in moderate yields with good selectivity.
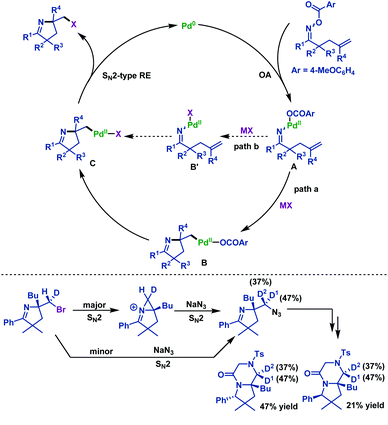
In 2015, Tong and co-workers65 described a palladium(0)-catalyzed imino-halogenation of γ,δ-unsaturated oxime esters with the assistance of different halide salts. A variety of synthetically useful 2-halomethyl pyrrolines were obtained in moderate to good yields (Scheme 36). The use of an excess amount of electron-poor phosphine ligands (P(4-F-C6H4)3) proved to be crucial to facilitate Calkyl–Pd(II)–halide reductive elimination. Compared to Bower's work,39–41,66 the pentafluorobenzoate leaving group was not necessary for this transformation. Moreover, the radical scavenger (TEMPO) did not have an adverse effect on the reaction, which indicated that the radical process was not involved in these transformations.
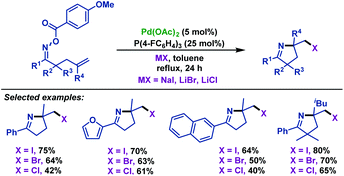 | ||
| Scheme 36 Tong's palladium-catalyzed imino-halogenation of γ,δ-unsaturated oxime esters with halide salts. | ||
A classical transition-metal-catalyzed pathway is documented in Scheme 37. First, the oxidative addition of γ,δ-unsaturated oxime esters to the Pd(0) catalyst affords imino-Pd(II) intermediate A, which is followed by alkene insertion to generate Calkyl–Pd(II) intermediate B (Scheme 37, path a). After ligand exchange with halide salts, B is converted into the Calkyl–Pd(II)–halide intermediate C. Alternatively, ligand exchange of intermediate A occurs before the alkene insertion (Scheme 37, path b). Finally, C undergoes Calkyl–Pd(II)–halide reductive elimination to deliver 2-halomethyl pyrrolines. Although path a is proved to be more reasonable, path b cannot be completely ruled out.
To get more insight into the unprecedented nature of Calkyl–Pd(II)–Br reductive elimination, the authors conducted further studies regarding the stereochemical nature of the C–Br bond forming step (Scheme 37). After a four-step synthetic transformation, two separable diastereomers were obtained and analyzed by NOE experiments. The NOE experiment results clearly indicated that Calkyl–Pd(II)–Br reductive elimination occurred via an SN2-type pathway, which was consistent with microscopic reversibility in the oxidative addition of alkyl halide to Pd(0) species.
Shortly after, the Bower group66 illustrated palladium-catalyzed imino-acylation, imino-arylation, imino-carboxylation, imino-alkynylation and imino-vinylation of γ,δ-unsaturated oxime esters with various nucleophiles (Scheme 38). The pentafluorobenzoate leaving group was found to be crucial for efficient cyclization. The reaction mechanism may involve the following steps: oxidative addition of Pd0 into the N–O bond of γ,δ-unsaturated oxime esters generates N–PdII intermediates, which undergo 5-exo-trig cyclization to form Calkyl–PdII intermediates. Then, Calkyl–PdII intermediates are intercepted by various nucleophiles under carbonylative or noncarbonylative conditions to provide diversiform functionalized pyrrolines.
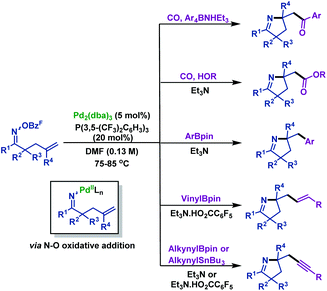 | ||
| Scheme 38 Bower's palladium-catalyzed imino-acylation, imino-arylation, imino-carboxylation, imino-alkynylation and imino-vinylation of γ,δ-unsaturated oxime esters with various nucleophiles. | ||
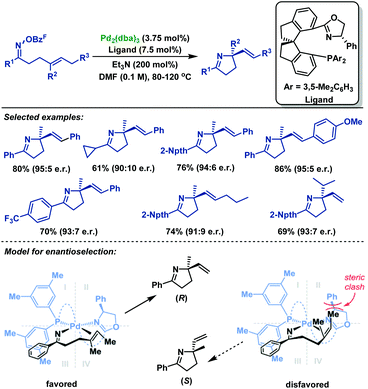
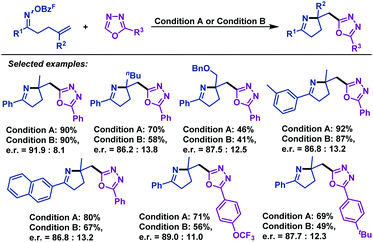
In 2017, Bower and co-workers67 realized the first example of highly enantioselective Narasaka–Heck cyclizations of γ,δ-unsaturated oxime esters (Scheme 39). The use of the SPINOL-derived P,N-ligand promotes Pd-catalyzed 5-exo-trig cyclization of a range of γ,δ-unsaturated oxime esters to generate pyrrolines containing tetrasubstituted nitrogen-bearing stereocenters in 56%–86% yields and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–95
10–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er. The structural features of the ligand backbone play a key role in enantioinduction. Besides, the key factor in the chemical efficiency of this ligand likely lies in the weak donor ability of the N atom of oxazoline, which enhances σ-donation from the trans-imino group. This lowers the basicity of the imino moiety, thus suppressing protodepalladation and the cyclization efficiency is enhanced.
5 er. The structural features of the ligand backbone play a key role in enantioinduction. Besides, the key factor in the chemical efficiency of this ligand likely lies in the weak donor ability of the N atom of oxazoline, which enhances σ-donation from the trans-imino group. This lowers the basicity of the imino moiety, thus suppressing protodepalladation and the cyclization efficiency is enhanced.
In 2019, the Liang group68 developed a palladium-catalyzed imino-polyfluorophenylation of γ,δ-unsaturated polyfluorobenzoyl oxime esters, which proceeds through iminopalladation and polyfluorobenzoyloxy decarboxylation cascade reaction, thus delivering various polyfluorophenylated pyrrolines (Scheme 40). This reaction effectively utilized the polyfluorobenzoyloxy leaving group as the polyfluorophenylated source instead of the use of additional polyfluoroarene, which provided a new atom-economic strategy to synthesize polyfluorophenylated pyrrolines. A plausible mechanism to account for the formation of polyfluorophenylated pyrrolines was proposed by the authors. The reaction is initiated by the oxidative addition of Pd0 to γ,δ-unsaturated oxime esters to afford PdII intermediate A, which is followed by alkene insertion to generate the Calkyl–PdII intermediate B. Subsequently, intermediate B could release a molecule of CO2 to form intermediate C by the decarboxylation of the pentafluorobenzoyloxy. Finally, intermediate C undergoes C–PdII–C reductive elimination to deliver polyfluorophenylated pyrrolines.
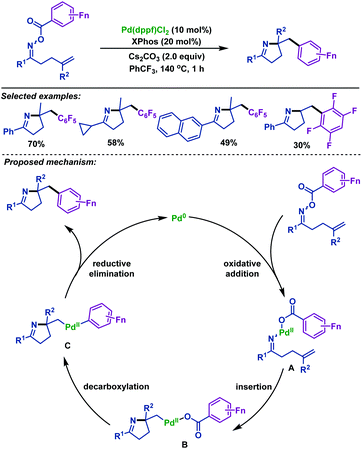 | ||
| Scheme 40 Liang's palladium-catalyzed imino-polyfluorophenylation of γ,δ-unsaturated polyfluorobenzoyl oxime esters. | ||
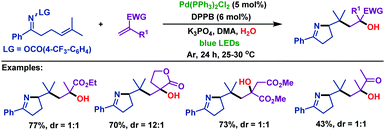
Very recently, Zhou and Xia69 described a palladium-catalyzed domino cross-coupling reaction of γ,δ-unsaturated oxime esters with aromatic alkenes (Scheme 41). This reaction was different from the previous palladium catalyzed reactions, in which the N–O bond was activated by photoexcited-state Pd(0). A wide range of functional groups were well tolerated in the reaction to afford the corresponding products in excellent yields. Control experiments demonstrated that both the SET pathway and oxidative addition were involved. The mechanism was proposed by the authors: first, oxidative addition of Pd(0) to the N–O bond of γ,δ-unsaturated oxime esters generates Pd(II) intermediate A, which is followed by 5-exo-trig cyclization to give alkyl radical intermediate B and Pd(I) species through a SET process. After radical addition to aromatic alkenes, conversion of B into radical intermediate C occurs. Subsequently, C coordinates with the palladium complex to deliver Pd(II) intermediate D, and then followed by β-H elimination to give the desired products.
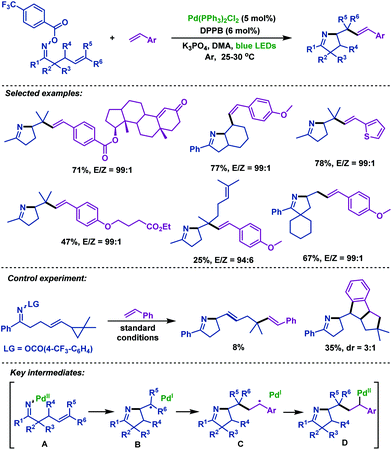 | ||
| Scheme 41 Zhou and Xia's palladium-catalyzed domino cross-coupling reaction of γ,δ-unsaturated oxime esters with aromatic alkenes under blue LED irradiation. | ||
Moreover, the palladium-catalyzed domino cross-coupling reaction of γ,δ-unsaturated oxime esters with α,β-unsaturated esters (ketones) was also investigated by Zhou and Xia (Scheme 42).69 The α,β-unsaturated esters (ketones) underwent 1,4-addition, which is followed by hydroxylation to form the corresponding α-OH esters (ketones).
2.3 Concurrence of N–O bond oxidative addition and N–O bond homolytic cleavage in cyclization reactions
A palladium-catalyzed enantioselective imino-arylation of γ,δ-unsaturated oxime esters with oxadiazoles was achieved by Zhu and colleagues in 2017 (Scheme 45).71 More importantly, in the presence of a chiral phosphine ligand (Synphos), the enantioselective version of this unprecedented transformation was subsequently realized. The corresponding pyrrolines were obtained in good yields with good enantioselectivity. Control experiments indicated the possible concurrence of single electron transfer and the two electron process in this Pd-catalyzed transformation.3. Cyclization reactions of other unsaturated oxime esters
3.1 Palladium-catalyzed reactions
In 2005, Ichikawa and co-workers72 realized an intramolecular Heck-type 5-endo-trig cyclization of β,γ-unsaturated oxime esters. A range of substituted 5-fluoro-3H-pyrroles can be generated in good yields using PPh3 as a ligand for Pd(0). This transformation started from the oxidative addition of N–O bonds, followed by 5-endo-trig cyclization and β-fluorine elimination (Scheme 46a). Moreover, in 2006 this group73 expanded the scope of substrates to 2-(trifluoromethyl)allyl ketone O-pentafluorobenzoyloximes, which underwent the similar 5-endo-trig cyclization, followed by β-F elimination to afford various 4-difluoro-methylene-1-pyrrolines (Scheme 46b).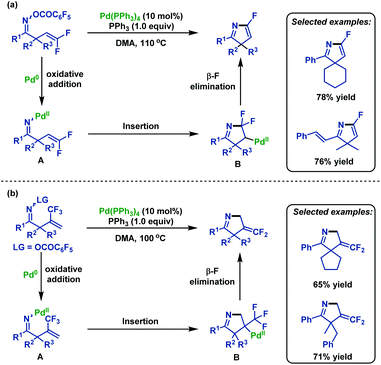 | ||
| Scheme 46 Ichikawa's palladium-catalyzed intramolecular Heck-type 5-endo-trig cyclization of β,γ-unsaturated oxime esters. | ||
3.2 Rhodium-catalyzed reactions
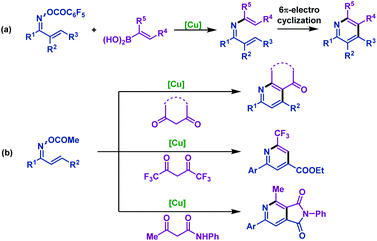
The first Rh(III)-catalyzed coupling of α,β-unsaturated oxime esters with alkenes was developed by Rovis and co-workers. In 2013, they reported74 a novel strategy to synthesize substituted pyridines from α,β-unsaturated oxime esters and activated alkenes in good yields and high regioselectivity. The oxime ester moieties were used as internal oxidants to complete the catalytic cycle. Mechanism investigations indicated that the substituted pyridines were formed via reversible C–H activation, alkene insertion, C–N bond formation and N–O bond cleavage processes. It is worth noting that the regioselectivity depends on the nature of alkenes and only activated alkenes react with exquisite regioselectivity (Scheme 47).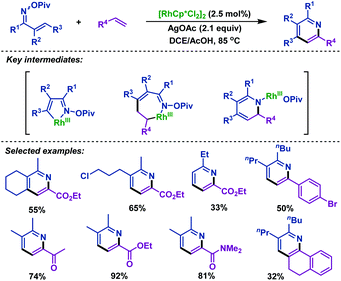 | ||
| Scheme 47 Rovis’ rhodium-catalyzed coupling of α,β-unsaturated oxime esters with alkenes to synthesize pyridines. | ||
In order to remove this restriction, the same group75 reported a Rh(III)-catalyzed decarboxylative coupling of α,β-unsaturated oxime esters with acrylic acids in 2014. In this transformation, the COOH moiety of acrylic acid served as a traceless activating group, which imparted the regioselectivity in the C–H activation process. Subsequently, the carboxylic acid moiety of acrylic acid derivatives can be removed by decarboxylation. Under the optimized conditions, diversiform substituted pyridines with good regioselectivity were obtained in good yields. Mechanistic studies indicated that the 6π-electrocyclization process can be ruled out (Scheme 48).
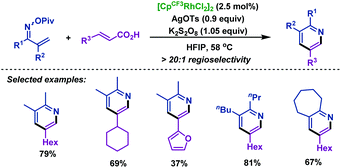 | ||
| Scheme 48 Rovis's rhodium-catalyzed decarboxylative coupling of acrylic acids with α,β-unsaturated oxime esters. | ||
In 2015, the Rovis group76 developed an efficient Rh(III)-catalyzed coupling of α,β-unsaturated oxime esters with 1,1-disubstituted alkenes for the synthesis of 2,3-dihydropyridines. The electron-deficient trifluoromethyl-substituted Cp*CF3 was found to be optimal to promote this transformation. Notably, catalytic reduction of the 2,3-dihydropyridines in one pot delivered piperidine derivatives with good diastereocontrol (Scheme 49).
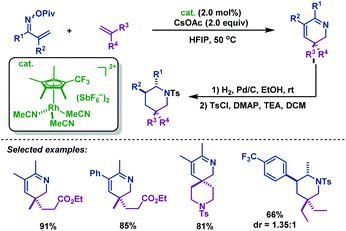 | ||
| Scheme 49 Rovis’ Rh(III)-catalyzed coupling of α,β-unsaturated oxime esters with 1,1-disubstituted alkenes for the synthesis of 2,3-dihydropyridines. | ||
3.3 Nickel-catalyzed reactions
In 2012, Kurahashi and Matsubara77 described a nickel-catalyzed cyclization of α,β-unsaturated oxime ethers with alkynes to synthesize 2,3,4,6-tetrasubstituted pyridines (Scheme 50). Different from the previous Rh-catalyzed cyclization reactions,74–76 this reaction was initiated by oxidative addition of Ni(0) to the N–O bond of α,β-unsaturated oxime ethers to afford intermediate A. Then, internal alkynes inserted into the N–Ni bond to afford intermediate B, followed by intramolecular insertion of the alkene to afford C. Subsequently, ligand exchange reaction of C with iPrOH afforded intermediate D, which is followed by two β-H elimination processes to give the final pyridine products.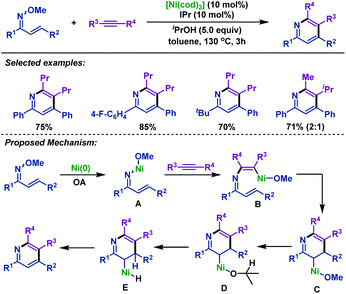 | ||
| Scheme 50 Kurahashi and Matsubara's nickel-catalyzed cyclization of α,β-unsaturated oxime ethers with alkynes to synthesize pyridines. | ||
It is noteworthy that the present nickel-catalyzed cyclization can be applied not only to α,β-unsaturated oxime ethers but also to β,γ-unsaturated oxime ethers for the synthesis of pyridines (Scheme 51).77 Diverse β,γ-unsaturated oxime ethers, bearing either electron-donating groups or electron-withdrawing groups, smoothly received cycloaddition with internal alkynes, delivering diversiform pyridines in good yields. Functional groups such as fluoro and methoxyl which are useful in further synthetic transformations were all well tolerated under the optimal conditions.
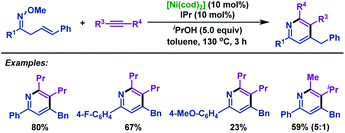 | ||
| Scheme 51 Kurahashi and Matsubara's nickel-catalyzed cyclization of β,γ-unsaturated oxime ethers with alkynes to synthesize pyridines. | ||
3.4 Copper-catalyzed reactions
In 2008, Liebeskind and colleagues78 disclosed a copper-catalyzed annulation of α,β-unsaturated oxime esters with alkenylboronic acids, which underwent oxidative addition, transmetallation and 6π-electrocyclization to synthesize various substituted pyridines (Scheme 52a). Recently, Guo and co-workers79 realized the copper-catalyzed [4 + 2] cycloaddition reaction of α,β-unsaturated oxime esters with 1,3-dicarbonyl compounds for the synthesis of three types of structurally diverse multisubstituted pyridines. The addition of the radical scavenger did not inhibit the reaction and the corresponding radical captured intermediate was not detected, which demonstrated the reaction proceeded through an ionic pathway rather than the radical process (Scheme 52b).Although the cyclization of unsaturated oxime esters mostly occurs via 5-exo processes, in 2014, the Guan group80 established a copper-catalyzed 5-endo-trig cyclization of β,γ-unsaturated oxime esters for the rapid construction of 2-arylpyrroles in moderate to good yields. This cyclization reaction features broad functional group tolerance. The authors proposed an anti-Baldwin 5-endo-trig radical cyclization pathway, and the iminyl radical intermediate was involved in the catalytic cycle (Scheme 53).
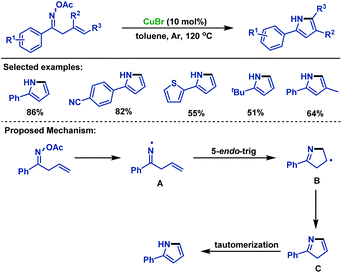 | ||
| Scheme 53 Guan's copper-catalyzed 5-endo-trig cyclization of β,γ-unsaturated oxime esters for the synthesis of pyrroles. | ||
4. Summary and outlook
We have summarized the cascade cyclization reactions of unsaturated oxime esters with various nucleophiles in the last decade. Related cyclizations as well as enantioselective Narasaka–Heck cyclizations are also included in this review. By using this protocol, a variety of functionalized pyrrolines and related scaffolds, including biologically significant N-heterocycles, have been efficiently accessed in moderate to good yields.Despite the huge progress made in this field, some challenges and opportunities still remain. For instance, is it possible to develop asymmetric reactions based on the radical species? Also further research on the synthesis of more diverse functionalized nitrogen-containing scaffolds should be expected. Moreover, some cascade reactions could be employed in this strategy for the synthesis of complex organic structures.
We hope this brief review will serve as a handy reference for organic chemists who are interested in the cascade cyclization of unsaturated oxime esters and for those who wish to utilize this important strategy for the synthesis of drug candidates and natural products.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We express our gratitude to the National Natural Science Foundation of China (No.: 21901184; 21572160; 21702091); the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (No.: 2018KJ159), the Doctoral Program Foundation of Tianjin Normal University (No.: 043135202-XB1703) and the Program for Innovative Research Team in University of Tianjin (No.: TD13-5074) for generous financial support.Notes and references
- E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, Rings in drugs, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed.
- http://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/fies/TopUSPharmaceuticalProductsof2013.pdf , accessed on 28th March 2017.
- I. V. Magedov, G. Luchetti, N. M. Evdokimov, M. Manpadi, W. F. A. Steelant, S. V. Slambrouck, P. Tongwa, M. Y. Antipin and A. Kornienko, Novel three-component synthesis and antiproliferative properties of diversely functionalized pyrrolines, Bioorg. Med. Chem. Lett., 2008, 18, 1392 CrossRef CAS PubMed.
- M. Biava, G. C. Porretta, G. Poce, S. Supino, D. Deidda, R. Pompei, P. Molicotti, F. Manetti and M. Botta, Antimycobacterial agents. Novel diarylpyrrole derivatives of BM212 endowed with high activity toward mycobacterium, tuberculosis and low cytotoxicity, J. Med. Chem., 2006, 49, 4946 CrossRef CAS PubMed.
- C. Marti and E. M. Carreira, Total synthesis of (−)-spirotryprostatin B: synthesis and related studies, J. Am. Chem. Soc., 2005, 127, 11505 CrossRef CAS PubMed.
- J. Boivin, A.-M. Schiano and S. Z. Zard, Iminyl radicals by stannane mediated cleavage of oxime esters, Tetrahedron Lett., 1994, 35, 249 CrossRef CAS.
- J. Boivin, A.-C. Callier-Dublanchet, B. Quiclet-Sire, A.-M. Schiano and S. Z. Zard, Iminyl, amidyl, and carbamyl radicals from O-benzoyl oximes and O-benzoyl hydroxamic acid derivatives, Tetrahedron, 1995, 51, 6517 CrossRef CAS.
- M. M. Jackman, Y. Cai and S. L. Castle, Recent advances in iminyl radical cyclizations, Synthesis, 2017, 49, 1785 CrossRef CAS.
- M. Kitamura and K. Narasaka, Catalytic radical cyclization of oximes induced by one-electron transfer, Bull. Chem. Soc. Jpn., 2008, 81, 539 CrossRef CAS.
- N. J. Race, I. R. Hazelden, A. Faulkner and J. F. Bower, Recent developments in the use of aza-Heck cyclizations for the synthesis of chiral N-heterocycles, Chem. Sci., 2017, 8, 5248 RSC.
- J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Hydroxylamine derivatives as nitrogen-radical precursors in visible-light photochemistry, Chem. – Eur. J., 2018, 24, 12154 CrossRef CAS PubMed.
- J. Li, Y. Hu, D. Zhang, Q. Liu, Y. Dong and H. Liu, Transition metal-catalyzed reactions involving oximes, Adv. Synth. Catal., 2017, 359, 710 CrossRef CAS.
- H. Huang, J. Cai and G.-J. Deng, O-Acyl oximes: versatile building blocks for N-heterocycle formation in recent transition metal catalysis, Org. Biomol. Chem., 2016, 14, 1519 RSC.
- M. Kitamura and K. Narasaka, Synthesis of aza-heterocycles from oximes by amino-heck reaction, Chem. Rec., 2002, 2, 268 CrossRef CAS PubMed.
- K. Narasaka and M. Kitamura, Amination with oximes, Eur. J. Org. Chem., 2005, 4505 CrossRef CAS.
- J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Visible light photoredox-controlled reactions of N-radicals and radical ions, Chem. Soc. Rev., 2016, 45, 2044 RSC.
- T. Xiong and Q. Zhang, New amination strategies based on nitrogen-centered radical chemistry, Chem. Soc. Rev., 2016, 45, 3069 RSC.
- Y. Zhao and W. Xia, Recent advances in radical-based C–N bond formation via photo-/electrochemistry, Chem. Soc. Rev., 2018, 47, 2591 RSC.
- J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Exploration of visible-light photocatalysis in heterocycle synthesis and functionalization: reaction design and beyond, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed.
- P. Xiong and H.-C. Xu, Chemistry with electrochemically generated N-centered radicals, Acc. Chem. Res., 2019, 52, 3339 CrossRef CAS PubMed.
- W. Yin and X. Wang, Recent advances in iminyl radical-mediated catalytic cyclizations and ring-opening reactions, New J. Chem., 2019, 43, 3254 RSC.
- T. Xiao, H. Huang, D. Anand and L. Zhou, Iminyl-radical-triggered C–C bond cleavage of cycloketone oxime derivatives: generation of distal cyano-substituted alkyl radicals and their functionalization, Synthesis, 2020, 52, 1585 CrossRef CAS.
- C. Song, X. Shen, F. Yu, Y.-P. He and S. Yu, Generation and application of iminyl radicals from oxime derivatives enabled by visible light photoredox catalysis, Chin. J. Org. Chem., 2020, 40 DOI:10.6023/cjoc202004008.
- X.-Y. Yu, J.-R. Chen, P.-Z. Wang, M.-N. Yang, D. Liang and W.-J. Xiao, A visible-light-driven iminyl radical-mediated C-C single bond cleavage/radical addition cascade of oxime esters, Angew. Chem., Int. Ed., 2018, 57, 738 CrossRef CAS PubMed.
- X.-Y. Yu, Q.-Q. Zhao, J. Chen, J.-R. Chen and W.-J. Xiao, Copper-catalyzed radical cross-coupling of redox-active oxime esters, styrenes, and boronic acids, Angew. Chem., Int. Ed., 2018, 57, 15505 CrossRef CAS PubMed.
- X.-Y. Yu, J. Chen, H.-W. Chen, W.-J. Xiao and J.-R. Chen, Visible-light-driven copper-catalyzed C(sp3)−O cross-coupling of benzylic radicals with phenols, Org. Lett., 2020, 22, 2333 CrossRef CAS PubMed.
- X.-Y. Yu, Q.-Q. Zhao, J. Chen, W.-J. Xiao and J.-R. Chen, When light meets nitrogen-centered radicals: from reagents to catalysts, Acc. Chem. Res., 2020, 53, 1066 CrossRef CAS PubMed.
- Z. Mirjafary, M. Abdoli, H. Saeidian, A. Kakanejadifard and S. M. F. Farnia, Review of the synthesis of acyclic and cyclic oxime ethers, RSC Adv., 2016, 6, 17740 RSC.
- Z. Mirjafary, M. Abdoli, H. Saeidian, S. Boroon and A. Kakanejadifard, Oxime ethers as versatile precursors in organic synthesis: a review, RSC Adv., 2015, 5, 79361 RSC.
- H. Huang, X. Ji, W. Wua and H. Jiang, Transition metal-catalyzed C–H functionalization of N-oxyenamine internal oxidants, Chem. Soc. Rev., 2015, 44, 1155 RSC.
- J. A. Blake, D. A. Pratt, S. Lin, J. C. Walton, P. Mulder and K. U. Ingold, Thermolyses of O-phenyl oxime ethers. A new source of iminyl radicals and a new source of aryloxyl radicals, J. Org. Chem., 2004, 69, 3112 CrossRef CAS.
- J. C. Walton, The oxime portmanteau motif: released heteroradicals undergo incisive EPR interrogation and deliver diverse heterocycles, Acc. Chem. Res., 2014, 47, 1406 CrossRef CAS PubMed.
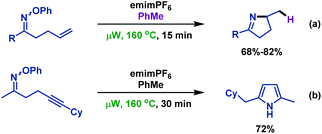
- F. Portela-Cubillo, J. S. Scottb and J. C. Walton, Microwave-assisted preparations of dihydropyrroles from alkenone O-phenyl oximes, Chem. Commun., 2007, 39, 4041 RSC.
- F. Portela-Cubillo, J. S. Scott and J. C. Walton, Microwave-assisted syntheses of N-heterocycles using alkenone-, alkynone- and aryl-carbonyl O-phenyl oximes: formal synthesis of neocryptolepine, J. Org. Chem., 2008, 73, 5558 CrossRef CAS PubMed.
- Y. Cai, A. Jalan, A. R. Kubosumi and S. L. Castle, Microwave-promoted tin-free iminyl radical cyclization with TEMPO trapping: a practical synthesis of 2-acylpyrroles, Org. Lett., 2015, 17, 488 CrossRef CAS PubMed.
- Y. Koganemaru, M. Kitamura and K. Narasaka, Synthesis of dihydropyrrole derivatives by copper-catalyzed cyclization of γ,δ-unsaturated ketone O-methoxycarbonyloximes, Chem. Lett., 2002, 31, 784 CrossRef.
- A. Faulkner, N. J. Race, J. S. Scott and J. F. Bower, Copper catalyzed Heck-like cyclizations of oxime esters, Chem. Sci., 2014, 5, 2416 RSC.
- A. Faulkner and J. F. Bower, Highly efficient narasaka–heck cyclizations mediated by P(3,5-(CF3)2C6H3)3: facile access to N-heterobicyclic scaffolds, Angew. Chem., Int. Ed., 2012, 51, 1675 CrossRef CAS PubMed.
- A. Faulkner, J. S. Scott and J. F. Bower, Palladium catalyzed cyclizations of oxime esters with 1, 1-disubstituted alkenes: synthesis of α, α-disubstituted dihydropyrroles and studies towards an asymmetric protocol, Chem. Commun., 2013, 49, 1521 RSC.
- N. J. Race and J. F. Bower, Palladium catalyzed cyclizations of oxime esters with 1,2-disubstituted alkenes: synthesis of dihydropyrroles, Org. Lett., 2013, 15, 4616 CrossRef CAS PubMed.
- K. Guo, H. Zhang, S. Cao, C. Gu, H. Zhou, J. Li and Y. Zhu, Copper-catalyzed domino cyclization/trifluoromethylthiolation of unactivated alkenes: access to SCF3-containing pyrrolines, Org. Lett., 2018, 20, 2261 CrossRef CAS PubMed.
- Y. Wang, J. Ding, J. Zhao, W. Sun, C. Lian, C. Chen and B. Zhu, Iminyl radical-promoted imino sulfonylation, imino cyanogenation and imino thiocyanation of γ,δ-unsaturated oxime esters: synthesis of versatile functionalized pyrrolines, Org. Chem. Front., 2019, 6, 2240 RSC.
- C. Chen, J. Ding, Y. Wang, X. Shi, D. Jiao and B. Zhu, Copper-catalyzed iminohalogenation of γ,δ-unsaturated oxime esters with halide salts: synthesis of 2-halomethyl pyrrolines, Tetrahedron Lett., 2019, 60, 151000 CrossRef.
- J. Zhao, Y. Bao, Y. Wang, C. Chen and B. Zhu, Copper-catalyzed group-transfer radical cyclization of γ,δ-unsaturated oxime esters: synthesis of ester-functionalized pyrrolines, Asian J. Org. Chem., 2019, 8, 1317 CrossRef CAS.
- L. Wang and C. Wang, Copper-catalyzed diamination of oxime ester-tethered unactivated alkenes with unprotected Amines, J. Org. Chem., 2019, 84, 6547 CrossRef CAS PubMed.
- H.-B. Yang and N. Selander, Divergent iron-catalyzed coupling of O-acyloximes with silyl enol ethers, Chem. – Eur. J., 2017, 23, 1779 CrossRef CAS PubMed.
- T. Shimbayashi, K. Okamoto and K. Ohe, Iron-catalyzed aminative cyclization/intermolecular homolytic aromatic substitution using oxime esters and simple arenes, Chem. – Asian J., 2018, 13, 395 CrossRef CAS PubMed.
- T. Shimbayashi, D. Nakamoto, K. Okamoto and K. Ohe, Facile construction of tetrahydropyrrolizines by iron-catalyzed double cyclization of alkene-tethered oxime esters with 1,2-disubstituted alkenes, Org. Lett., 2018, 20, 3044 CrossRef CAS.
- J. Boivin, A.-M. Schiano, S. Z. Zard and H. Zhang, A new method for the generation and capture of iminylradicals, Tetrahedron Lett., 1999, 40, 4531 CrossRef CAS.
- H.-B. Yang, S. R. Pathipati and N. Selander, Nickel-catalyzed 1,2-aminoarylation of oxime ester-tethered alkenes with boronic acids, ACS Catal., 2017, 7, 8441 CrossRef CAS.
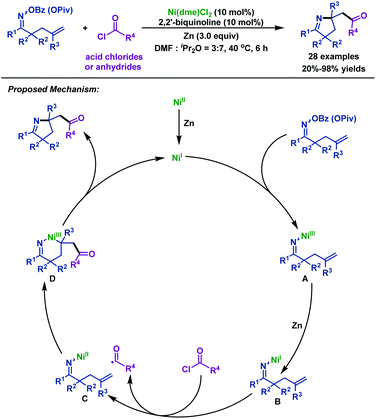
- L. Wang and C. Wang, Ni-catalyzed 1,2-iminoacylation of alkenes via a reductive strategy, Org. Chem. Front., 2018, 5, 3476 RSC.
- N. J. Race, A. Faulkner, M. H. Shaw and J. F. Bower, Dichotomous mechanistic behavior in Narasaka–Heck cyclizations: electron rich Pd-catalysts generate iminyl radicals, Chem. Sci., 2016, 7, 1508 RSC.
- C. Chen, Y. Bao, J. Zhao and B. Zhu, Silver-promoted cascade radical cyclization of γ, δ-unsaturated oxime esters with P(O)H compounds: synthesis of phosphorylated pyrrolines, Chem. Commun., 2019, 55, 14697 RSC.
- J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Visible-light-mediated generation of nitrogen-centered radicals: metal-free hydroimination and iminohydroxylation cyclization reactions, Angew. Chem., Int. Ed., 2015, 54, 14017 CrossRef CAS PubMed.
- S.-H. Cai, J.-H. Xie, S. Song, L. Ye, C. Feng and T.-P. Loh, Visible-light-promoted carboimination of unactivated alkenes for the synthesis of densely functionalized pyrroline derivatives, ACS Catal., 2016, 6, 5571 CrossRef CAS.
- R. Mao, Z. Yuan, Y. Li and J. Wu, N-Radical-initiated cyclization through insertion of sulfur dioxide under photoinduced catalyst-free conditions, Chem. – Eur. J., 2017, 23, 8176 CrossRef CAS PubMed.
- H. Jiang and A. Studer, Iminyl-radicals by oxidation of α-imino-oxy acids: photoredox-neutral alkene carboimination for the synthesis of pyrrolines, Angew. Chem., Int. Ed., 2017, 56, 12273 CrossRef CAS PubMed.
- J. Davies, N. S. Sheikh and D. Leonori, Photoredox imino functionalizations of olefins, Angew. Chem., Int. Ed., 2017, 56, 13361 CrossRef CAS PubMed.
- L. Tu, J.-L. Liu, W. Tang, M. Su and F. Liu, Radical aza-cyclization of α–imino-oxy acids for synthesis of alkene-containing N–heterocycles via dual cobaloxime and photoredox catalysis, Org. Lett., 2020, 22, 1222 CrossRef PubMed.
- K. Usami, E. Yamaguchi, N. Tada and A. Itoh, Visible-light-mediated iminyl radical generation from benzyl oxime ether: synthesis of pyrroline via hydroimination cyclization, Org. Lett., 2018, 20, 5714 CrossRef CAS PubMed.
- S.-H. Cai, D.-X. Wang, L. Ye, Z.-Y. Liu, C. Feng and T.-P. Loh, Pyrroline synthesis via visible-light-promoted hydroimination of unactivated alkenes with N,N’-dimethylpropylene urea as H-donor, Adv. Synth. Catal., 2018, 360, 1262 CrossRef CAS.
- F. Huang and S. Zhang, Iminyl radicals by reductive cleavage of N–O bond in oxime ether promoted by SmI2: a straightforward synthesis of five-membered cyclic imines, Org. Lett., 2019, 21, 7430 CrossRef CAS PubMed.
- H. Tsutsui and K. Narasaka, Synthesis of pyrrole derivatives by the Heck-type cyclization of γ,δ-unsaturated ketone O-pentafluorobenzoyloximes, Chem. Lett., 1999, 28, 45 CrossRef.
- C. Chen, L. Hou, M. Cheng, J. Su and X. Tong, Palladium(0)-catalyzed iminohalogenation of alkenes: synthesis of 2-halomethyl dihydropyrroles and mechanistic insights into the alkyl halide bond formation, Angew. Chem., Int. Ed., 2015, 54, 3092 CrossRef CAS PubMed.
- A. Faulkner, J. S. Scott and J. F. Bower, An umpolung approach to alkene carboamination: palladium catalyzed 1, 2-amino-acylation, -carboxylation, -arylation, -vinylation, and -alkynylation, J. Am. Chem. Soc., 2015, 137, 7224 CrossRef CAS PubMed.
- N. J. Race, A. Faulkner, G. Fumagalli, T. Yamauchi, J. S. Scott, M. Rydén-Landergren, H. A. Sparkes and J. F. Bower, Enantioselective Narasaka–Heck cyclizations: synthesis of tetrasubstituted nitrogen-bearing stereocenters, Chem. Sci., 2017, 8, 1981 RSC.
- W.-X. Wei, S. Chen, Y. Xia, M. Li, X.-S. Li, Y.-P. Han, C.-T. Wang and Y.-M. Liang, Palladium-catalyzed intramolecular self-alkylation of polyfluoroarene via heck and decarboxylation process, ChemCatChem, 2019, 11, 5754 CrossRef CAS.
- L. Feng, L. Guo, C. Yang, J. Zhou and W. Xia, Visible-light-induced palladium-catalyzed intermolecular Narasaka–Heck reaction at room temperature, Org. Lett., 2020, 22, 3964 CrossRef CAS PubMed.
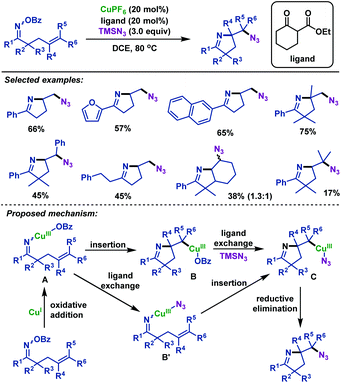
- H. Su, W. Li, Z. Xuan and W. Yu, Copper-catalyzed cyclization and azidation of γ,δ-unsaturated ketone O-benzoyl oximes, Adv. Synth. Catal., 2015, 357, 64 CrossRef CAS.
- X. Bao, Q. Wang and J. Zhu, Palladium-catalyzed enantioselective Narasaka–Heck reaction/direct C–H alkylation of arenes: iminoarylation of alkenes, Angew. Chem., Int. Ed., 2017, 56, 9577 CrossRef CAS PubMed.
- K. Sakoda, J. Mihara and J. Ichikawa, Heck-type 5-endo-trigcyclization promoted by vinylic fluorines: synthesis of 5-fluoro-3H-pyrroles, Chem. Commun., 2005, 37, 4684 RSC.
- J. Ichikawa, R. Nadano and N. Ito, 5-endo Heck-type cyclization of 2-(trifluoromethyl)allyl ketoneoximes: synthesis of 4-difluoromethylene-substituted 1-pyrrolines, Chem. Commun., 2006, 42, 4425 RSC.
- J. M. Neely and T. Rovis, Rh(III)-Catalyzed regioselective synthesis of pyridines from alkenes and α,β-unsaturated oxime esters, J. Am. Chem. Soc., 2013, 135, 66 CrossRef CAS PubMed.
- J. M. Neely and T. Rovis, Rh(III)-Catalyzed decarboxylative coupling of acrylic acids with unsaturated oxime esters: carboxylic acids serve as traceless activators, J. Am. Chem. Soc., 2014, 136, 2735 CrossRef CAS PubMed.
- F. Romanov-Michailidis, K. F. Sedillo, J. M. Neely and T. Rovis, Expedient access to 2,3-dihydropyridines from unsaturated oximes by Rh(III)-catalyzed C–H activation, J. Am. Chem. Soc., 2015, 137, 8892 CrossRef CAS PubMed.
- Y. Yoshida, T. Kurahashi and S. Matsubara, Nickel-catalyzed cycloaddition of α,β-unsaturated oximes with alkynes: synthesis of highly substituted pyridine derivatives, Chem. Lett., 2012, 41, 1498 CrossRef CAS.
- S. Liu and L. S. Liebeskind, A simple, modular synthesis of substituted pyridines, J. Am. Chem. Soc., 2008, 130, 6918 CrossRef CAS PubMed.
- L. Zhang, J. Duan, G. Xu, X. Ding, Y. Mao, B. Rong, N. Zhu, Z. Fang, Z. Li and K. Guo, Copper-catalyzed N–O cleavage of α,β-unsaturated ketoxime acetates toward structurally diverse pyridines, J. Org. Chem., 2020, 85, 2532 CrossRef CAS PubMed.
- W. Du, M.-N. Zhao, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Copper-catalyzed 5-endo-trig cyclization of ketoxime carboxylates: a facile synthesis of 2-arylpyrroles, Chem. Commun., 2014, 50, 7437 RSC.
| This journal is © the Partner Organisations 2020 |