Asymmetric organocatalytic double 1,6-addition: rapid access to chiral chromans with molecular complexity†
Abstract
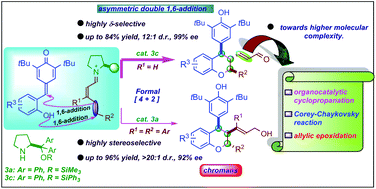
An organocatalytic cascade vinylogous double 1,6-addition strategy for the synthesis of chiral chroman derivatives is reported. The cascade reaction follows oxa-Michael/1,6-addition reactions between ortho-hydroxyphenyl-substituted para-quinone methides and 2,4-dienal derivatives to deliver chroman derivatives with excellent yields (up to 96%) and stereoselectivities (up to >20 : 1 dr, >99% ee). The chiral secondary amine-based Jørgensen–Hayashi organocatalysts are shown to catalyze the transformation of unbiased 2,4-dienals under mild reaction conditions with exclusive δ-site selectivity and high control over enantioselectivity at a remote position. The synthetic transformations of the products ensure molecular complexity with a great level of enantiocontrol.


 Please wait while we load your content...
Please wait while we load your content...