Aromatization-driven deconstruction/refunctionalization of unstrained rings
Fangzhi
Hu
 a,
Liang
Wang
a,
Liang
Wang
 ab,
Lubin
Xu
a and
Shuai-Shuai
Li
ab,
Lubin
Xu
a and
Shuai-Shuai
Li
 *ab
*ab
aCollege of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao 266109, China. E-mail: flyshuaishuai@126.com
bCollege of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Zhengzhou Rd. #53, Qingdao 266042, P. R. China
First published on 1st June 2020
Abstract
Aromatization-driven ring-opening/functionalization of common unstrained rings has been developed with the in situ generation of pre-aromatic fused spiro heterocycles as the key step, featuring (1) simple operation via a convenient one-pot reaction and (2) broad scope of various ring systems which do not require pre-activation.
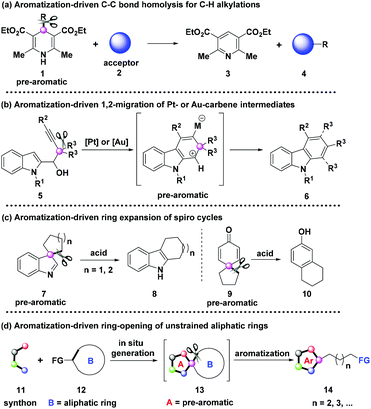
As is well known, aromatization is an important thermodynamic driving force for the formation of stable aromatic rings in organic synthesis.1 And the C–C bond activation as a significant route for molecular modification is a hot research field.2 Remarkably, the exploitation of aromatization-driven C–C bond cleavage can be traced back to 1972 with the assistance of transition metals.3 However, since then, the utilization of aromatization as a driving force for C–C bond cleavage has been unappreciated for a long time, and only a few relevant studies have been reported.3,4 Recently, this strategy has been revived gradually for elaborate transformations and has drawn increasing attention from chemists.5–8 For example, Melchiorre,6a Molander,6b and Chen6c developed C–H alkylation reactions independently by employing the aromatization-driven C–C bond homolysis strategy of 4-alkyl-1,4-dihydropyridines 1 (Scheme 1a). The Ma group performed the pioneering work on the aromatization-driven 1,2-migration of Pt- or Au-carbene intermediates for the synthesis of carbazoles (Scheme 1b).7 Besides, You8a–e and other groups8f–g reported the aromatization-driven ring-expanding rearomatization of spiroindolenines 7 for the construction of polycyclic indoles 8via acid-mediated migration, respectively. In addition, the spirocyclization-dienone-phenol rearrangement cascade reactions have been reported as well with the promotion of aromatization (Scheme 1c).9 Yet the major reason that restricts the wide application of aromatization as a driving force for synthetic transformations is the difficulty in the in situ generation of pre-aromatic substrates, which usually need to be prefabricated through tedious steps.
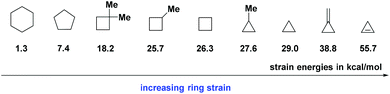
Aliphatic rings are ubiquitous in various kinds of organic compounds including pharmaceutical drugs, natural products, and functional materials.10 Thus, employing the widely-sourced aliphatic rings as starting materials for deconstruction and re-functionalization would be significantly important for the development of organic synthesis and industrial production. However, for a long time, chemists have been limited in the cleavage of strained rings, which were equipped with an inherent thermodynamic driving force for releasing the ring strain (Fig. 1).11 The C–C bond cleavage/editing of unstrained aliphatic rings is a compelling challenge owing to the high C–C bond dissociation energy.
This highlight article aims to provide a concise overview of the aromatization-driven ring deconstruction strategy of readily available unstrained cycloalkanones and cycloalkanamines via radical-mediated C–C bond fragmentation. The protocol features the in situ generation of pre-aromatic fused spiro cycles 13, as shown in Scheme 1d, which might bring a distinct research direction for the development of organic chemistry.
Aromatization-driven C–C bond cleavage of unstrained cycloalkanones
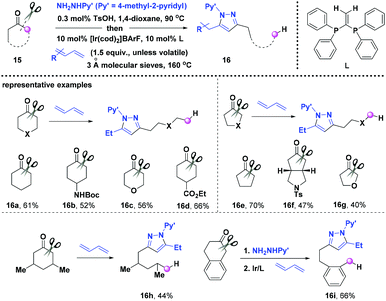
C–C bond activation/functionalization of cycloalkanones emerged as a useful method for synthesizing complex scaffolds. Seminal work by Jun developed the rhodium(I)-catalyzed ring-opening of medium to large cycloalkanone imines to provide various aliphatic chain decorated ketones in 2001.12 Afterwards, the pursuit for the C–C bond activation/functionalization of cycloalkanones went on uninterrupted, but only sporadic works were reported for unstrained ring activation.13 Among them, the Dong group has made outstanding contributions to the transition-metal catalyzed unstrained C–C bond activation with the assistance of in situ formed directing groups.13b–eVery recently, Dong and co-workers reported the efficient ring-opening reactions of unstrained cycloalkanones based on radical fragmentation involved aromatization-driven C–C bond activation (Scheme 2).14 These deacylative transformations provided various skeleton-functionalized aliphatic chains linking pyrazole via a three-component coupling in the presence of an iridium/phosphine combination. Considering the less-accessible carbocyclic pre-aromatics, the authors designed the three component involved 1,3-dipolar addition to prepare the precursor of the pre-aromatized heterocycle which serves as the key intermediate for the subsequent conversion. Various cycloalkanones 15 with different substitutions and ring sizes are available for the aromatization-driven deconstructive transformations. In addition, good regioselectivity has been observed when unsymmetrical ketones and heterocyclic ketones were used, and the bond scission preferentially occurred at more substituted carbons or the α-position of heteroatoms. Besides, various natural products were well tolerated for the C–C bond transformation.
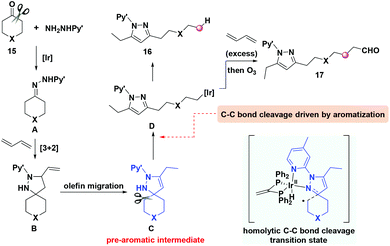
Based on mechanistic studies and DFT calculations, an aromatization-promoted homolytic C–C cleavage/radical recombination mechanism was proposed (Scheme 3). The initial [3 + 2] cycloaddition occurs between 1,3-butadiene and the hydrazone intermediate A, generating cyclic adduct B which could be isolated. Then olefin migration takes place to afford the dihydropyrazole intermediate C which serves as the pre-aromatic substrate to drive the following homolytic C–C bond cleavage with the promotion of an iridium catalyst. The control experiments indicate that no C–C cleavage occurs without the endocyclic double bond or the five-membered ring structure, which demonstrates the significant role of the intermediate C. Subsequently, the aromatization-driven deconstruction of the unstrained ring occurs, providing the iridium complex D bearing aromatized pyrazole. Then the C–H reductive elimination or coupling with 1,3-butadiene of iridium complex D takes place to afford the corresponding products 16 and 17, respectively. In addition to cycloalkanones, the deacylative transformations are suitable for a variety of linear ketones as well, which offer strategic bond disconnections for editing of the molecular skeleton.
In addition, Fan and co-workers described the ring-opening reactions through [3 + 2] cycloaddition of enone with unstrained cyclic ketone hydrazone followed by an aromatization-driven homolytic C–C bond cleavage/radical reorganization (Scheme 4).15 In this reaction, the in situ generated pre-aromatic I is considered as the key intermediate likewise to drive the subsequent C–C bond disconnection for aromatization.
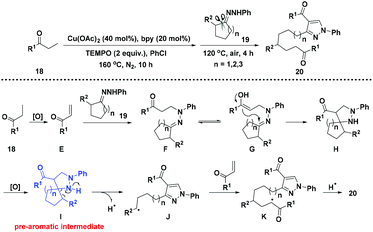 | ||
| Scheme 4 Ring-opening reactions for the synthesis of 4-acylpyrazole from unstrained cyclic ketone hydrazine. | ||
Aromatization-driven C–C bond cleavage of unstrained cycloalkanamines
Cycloalkanamines as essential structural units are widely found in natural products, pharmaceuticals, and agrochemicals. The development of convenient methods to make use of the widespread cycloalkanamines is extremely meaningful for modern chemical production. At present, the utilization of cycloalkanamines via ring-opening reconstruction is underdeveloped, and the scarce cases that are reported are almost limited to strained rings.16For example, Zheng and co-workers conducted the pioneering work on the photogenerated amine radical cation-involved ring-extension reactions of cyclopropyl- and cyclobutyl-anilines with alkenes and alkynes.16a–d Recently, Waser and co-workers described an oxidative ring-opening strategy to transform aminocyclopropanes into 1,3-dielectrophilic carbon intermediates bearing a halide atom (Br, I) and a N,O-acetal which could be converted into a wide range of α,γ-difunctionalized amines in a one-pot or two-step operation.16e
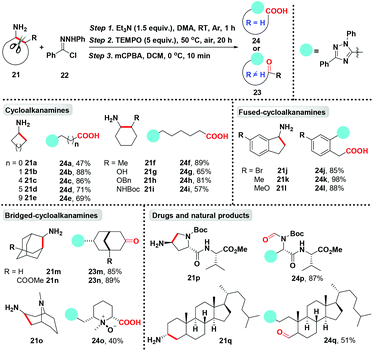
Despite the developments mentioned above on the application of cycloalkanamines as building blocks through ring-reconstruction and functionalization, ring-opening of unstrained cycloalkanamines still remains a compelling challenge. This situation can be attributed to ring-strain release as a thermodynamic driving force for strained cycloalkanamines, and the high reverse exo-cyclization rate constant of nitrile and imine for unstrained cycloalkanamines, especially for 5- or 6-membered cycloalkanamines.11,17 In this context, Han and co-workers achieved the introduction of aromatization as both dynamic and thermodynamic driving forces for driving ring-opening of unstrained primary cycloalkanamines.18 In this metal-free reaction, the deconstruction/functionalization of unstrained primary cycloalkanamines has been developed unprecedentedly, producing carbonyl compound tethered aliphatic chains and 1,2,4-triazole directly through the autooxidative aromatization-driven C(sp3)–C(sp3) bond cleavage (Scheme 5). Similarly, the C–C bond cleavage of cycloalkanamines preferentially at more substituted carbons or the α-position of heteroatoms was achieved. The wide substrate scope tolerance of this protocol has been revealed by meticulous evaluations, including monocyclic, bicyclic, bridged, and complex natural product derivatives containing primary cycloalkanamine moieties, which indicated its potential application in the pharmaceutical and chemical industries. In addition, the in situ generation of the pre-aromatic heterocycle remains the core of this transformation, which is consistent with Dong and Fan's work.
In the proposed catalytic cycle (Scheme 6), the initial nucleophilic substitution occurs between cycloalkanamine 21 and hydrazonyl chloride 22 to produce hydrazonamide L which is auto-oxidized by air to generate aminyl radical M. Then radical M undergoes the tandem 1,5-hydrogen atom shift/further oxidation/annulation process to form the key pre-aromatic heterocycle N, which could be isolated. The spiro heterocycle N tends to be oxidized by air to afford the cyclic amino radical intermediate O which appears in many N-containing heteroaryl migration reactions.19 Then aromatization serves as a driving force to promote the radical C–C bond cleavage to furnish the aromatic 1,2,4-triazole. The generated distal alkyl radical P linking aromatic 1,2,4-triazole is immediately intercepted by TEMPO to give the ring-opening product Q. The final acyclic carbonyl compounds 23 and 24 carrying 1,2,4-triazole could be obtained by the treatment of mCPBA via the oxidation/Cope-elimination sequence.
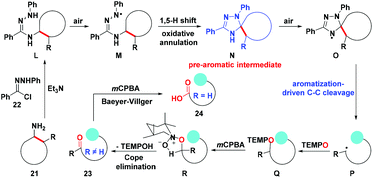 | ||
| Scheme 6 Proposed mechanism for the deconstruction/functionalization of unstrained primary cycloalkanamines. | ||
Conclusions
The C–C bond disconnection is of significant importance for editing the molecular skeleton in organic synthesis, especially for the cleavage of ubiquitous unstrained aliphatic rings. Aromatization as both dynamic and thermodynamic driving forces for promoting C–C bond cleavage could compensate for the chemical inertness of unstrained aliphatic rings. We have highlighted the aromatization-driven homolytic C–C bond cleavage of common unstrained rings promoted by the in situ generated pre-aromatic fused spiro cycles, which featured (1) simple operation via a convenient one-pot reaction and (2) broad scope of various ring systems which do not require pre-activation. From our viewpoint, the aromatization-driven deconstruction/refunctionalization of organic molecules would be a powerful toolbox for modifying molecular skeletons and enriching the diversity of molecules.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the NSFC (21978144, 21702117, and 21776148), the Key Research & Development Program of Shandong Province (2018GSF118224, 2019GSF108020, 2019GGXI02036, and 2019RKB01027), the Support Plan on Science and Technology for Youth Innovation of Universities in Shandong Province (2019KJM002) and the Opening Funding of Qingdao University of Science & Technology (QUSTHX201916 and QUSTHX202004). The Central Laboratory of Qingdao Agricultural University is also gratefully acknowledged for NMR determination.Notes and references
- (a) P. V. R. Schleyer and F. Pühlhofer, Recommendations for the evaluation of aromatic stabilization energies, Org. Lett., 2002, 4, 2873–2876 CrossRef CAS PubMed; (b) M. A. Halcrow, F. Urbanos and B. Chaudret, Aromatization of the B-ring of 5,7-dienyl steroids by the electrophilic ruthenium fragment “[Cp*Ru]+”, Organometallics, 1993, 12, 955–957 CrossRef CAS.
- (a) M. Murakami and Y. Ito, Cleavage of carbon–carbon single bonds by transition metals, Top. Organomet. Chem., 1999, 3, 97–129 CrossRef CAS; (b) F. Chen, T. Wang and N. Jiao, Recent advances in transition-metal-catalyzed functionalization of unstrained carbon-carbon bonds, Chem. Rev., 2014, 114, 8613–8661 CrossRef CAS PubMed; (c) D.-S. Kim, W.-J. Park and C.-H. Jun, Metal–organic cooperative catalysis in C–H and C–C bond activation, Chem. Rev., 2017, 117, 8977–9015 CrossRef CAS PubMed; (d) F. Song, T. Gou, B.-Q. Wang and Z.-J. Shi, Catalytic activations of unstrained C–C bond involving organometallic intermediates, Chem. Soc. Rev., 2018, 47, 7078–7115 RSC; (e) X. Wu and C. Zhu, Recent advances in radical-mediated C-C bond fragmentation of non-strained molecules, Chin. J. Chem., 2019, 37, 171–182 CAS; (f) C–C bond activation, Top. Curr. Chem., ed. G. Dong, Springer, Berlin, 2014, vol. 346, pp. 1–262 Search PubMed; (g) P.-h. Chen, B. A. Billett, T. Tsukamoto and G. Dong, “Cut and Sew” Transformations via transition-metal-catalyzed carbon−carbon bond activation, ACS Catal., 2017, 7, 1340–1360 CrossRef CAS PubMed; (h) L. Deng and G. Dong, Carbon-Carbon bond activation of ketones, Trends Chem., 2020, 2, 183–198 CrossRef.
- R. B. King and A. Efraty, Pentamethylcyclopentadienyl derivatives of transition metals. II. Synthesis of pentamethylcyclopentadienyl metal carbonyls from 5-acetyl-1,2,3,4,5-pentamethylcyclopentadiene, J. Am. Chem. Soc., 1972, 94, 3773–3779 CrossRef CAS.
- (a) R. H. Crabtree, R. P. Dion, D. J. Gibboni, D. V. Mcgrath and E. M. Holt, Carbon-carbon bond cleavage in hydrocarbons by iridium complexes, J. Am. Chem. Soc., 1986, 108, 7222–7227 CrossRef CAS; (b) G. Smits, B. Audic, M. D. Wodrich, C. Corminboeuf and N. Cramer, A β-Carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes, Chem. Sci., 2017, 8, 7174–7179 RSC.
- (a) J. Guin, C. Muck-Lichtenfeld, S. Grimme and A. Studer, Radical transfer hydroamination with aminated cyclohexadienes using polarity reversal catalysis: scope and limitations, J. Am. Chem. Soc., 2007, 129, 4498–4503 CrossRef CAS PubMed; (b) S. W. Youn, B. S. Kim and A. R. Jagdale, Pd-Catalyzed sequential C–C bond formation and cleavage: evidence for an unexpected generation of arylpalladium(II) species, J. Am. Chem. Soc., 2012, 134, 11308–11311 CrossRef CAS PubMed.
- (a) L. Buzzetti, A. Prieto, S. R. Roy and P. Melchiorre, Radical-based C-C bond-forming processes enabled by the photoexcitation of 4-alkyl-1,4-dihydropyridines, Angew. Chem., 2017, 129, 15235–15239 CrossRef; (b) á. Gutierrez-Bonet, C. Remeur, J. K. Matsui and G. A. Molander, Late-stage C–H alkylation of heterocycles and 1,4-quinones via oxidative homolysis of 1,4-dihydropyridines, J. Am. Chem. Soc., 2017, 139, 12251–12258 CrossRef PubMed; (c) X. Chen, F. Ye, X. Luo, X. Liu, J. Zhao, S. Wang, Q. Zhou, G. Chen and P. Wang, Histidine-specific peptide modification via visible-light-promoted C–H alkylation, J. Am. Chem. Soc., 2019, 141, 18230–18237 CrossRef CAS PubMed.
- (a) Y. Qiu, D. Ma, W. Kong, C. Fu and S. Ma, Regiocontrolled 1,2-migration in cyclization of 1-(indol-2-yl)-3-alkyn-1-ols: (Ph3P)Au+vs. PtCl4, Org. Chem. Front., 2014, 1, 62–67 RSC; (b) W. Kong, Y. Qiu, X. Zhang, C. Fu and S. Ma, Exclusive 1,2-aryl shift in platinum(II) chloride-catalyzed cyclization of 1-(Indol-2-yl)-2,3-allenols, Adv. Synth. Catal., 2012, 354, 2339–2347 CrossRef CAS; (c) W. Kong, C. Fu and S. Ma, Efficient synthesis of carbazoles via PtCl2-catalyzed RT cyclization of 1-(indol-2-yl)-2,3-allenols: scope and mechanism, Org. Biomol. Chem., 2012, 10, 2164–2173 RSC; (d) W. Kong, C. Fu and S. Ma, General Au-catalyzed benzannulation towards naturally occurring carbazole alkaloids from methoxypropadiene, Chem. – Eur. J., 2011, 17, 13134–13137 CrossRef CAS PubMed; (e) W. Kong, C. Fu and S. Ma, An efficient synthesis of carbazoles from PtCl2-catalyzed cyclization of 1-(indol-2-yl)-2,3-allenols, Chem. Commun., 2009, 4572–4574 RSC; (f) X. Zhang and S. Ma, Transition metal-catalyzed benzannulation towards naturally occurring carbazole alkaloids, Isr. J. Chem., 2018, 58, 608–621 CrossRef CAS.
- (a) Y. Wang, C. Zheng and S.-L. You, Iridium-catalyzed asymmetric allylic dearomatization by a desymmetrization strategy, Angew. Chem., Int. Ed., 2017, 56, 15093–15097 CrossRef CAS PubMed; (b) Q.-F. Wu, C. Zheng and S.-L. You, Enantioselective synthesis of spiro cyclopentane-1,3′-indoles and 2,3,4,9-tetrahydro-1H-carbazoles by Iridium-catalyzed allylic dearomatization and stereospecific migration, Angew. Chem., Int. Ed., 2012, 51, 1680–1683 CrossRef CAS PubMed; (c) C.-X. Zhuo, Q. Cheng, W.-B. Liu, Q. Zhao and S.-L. You, Enantioselective synthesis of pyrrole-based spiro- and polycyclic derivatives by Iridium-catalyzed asymmetric allylic dearomatization and controllable migration reactions, Angew. Chem., Int. Ed., 2015, 54, 8475–8479 CrossRef CAS PubMed; (d) C.-X. Zhuo, Y. Zhou, Q. Cheng, L. Huang and S.-L. You, Enantioselective construction of spiroindolines with three contiguous stereogenic centers and chiral tryptamine derivatives via reactive spiroindolenine intermediates, Angew. Chem., Int. Ed., 2015, 54, 14146–14149 CrossRef CAS PubMed; (e) C. Zheng, Z.-L. Xia and S.-L. You, Unified mechanistic understandings of Pictet-Spengler reactions, Chem, 2018, 4, 1952–1966 CrossRef CAS; (f) V. Gobé, V. Gandon and X. Guinchard, Reactions involving tryptamines and δ-allenyl aldehydes: competition between Pictet-Spengler reaction and cyclization to 1-aminotetralins, Adv. Synth. Catal., 2018, 360, 1280–1288 CrossRef; (g) G. Bai, F. Dong, L. Xu, Y. Liu, L. Wang and S.-S. Li, Controllable syntheses of spiroindolenines and senzazepinoindoles via hexafluoroisopropanol-mediated Redox-Neutral cascade process, Org. Lett., 2019, 21, 6225–6230 CrossRef CAS PubMed.
- (a) M. Yoshida, T. Nozaki, T. Nemoto and Y. Hamada, Formal meta-specific intramolecular Friedel–Crafts allylic alkylation of phenols through a spirocyclization–dienone–phenol rearrangement cascade, Tetrahedron, 2013, 69, 9609–9615 CrossRef CAS; (b) R. A. Bauer, T. A. Wenderski and D. S. Tan, Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion, Nat. Chem. Biol., 2013, 9, 21–29 CrossRef CAS PubMed.
- (a) Biologically Active Natural Products: Pharmaceuticals, ed. J. C. Stephen and H. G. Cutler, CRC Press, Boca Raton, FL, 2000 Search PubMed; (b) Comprehensive natural products II: Chemistry and Biology, ed. L. Mander and H.-W. Liu, Elsevier, Amsterdam, 2010 Search PubMed.
- For reviews on ring-opening of strained rings, see: (a) G. Fumagalli, S. Stanton and J. F. Bower, Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes, Chem. Rev., 2017, 117, 9404–9432 CrossRef CAS PubMed; (b) M. Murakami and N. Ishida, Potential of metal-catalyzed C–C single bond cleavage for organic synthesis, J. Am. Chem. Soc., 2016, 138, 13759–13769 CrossRef CAS PubMed; (c) L. Souillart and N. Cramer, Catalytic C–C bond activations via oxidative addition to transition metals, Chem. Rev., 2015, 115, 9410–9464 CrossRef CAS PubMed; (d) F. Wang, S. Yu and X. Li, Transition metal-catalysed couplings between arenes and strained or reactive rings: combination of C–H activation and ring scission, Chem. Soc. Rev., 2016, 45, 6462–6477 RSC.
- C.-H. Jun, H. Lee and S.-G. Lim, The C−C bond activation and skeletal rearrangement of cycloalkanone imine by Rh(I) catalysts, J. Am. Chem. Soc., 2001, 123, 751–752 CrossRef CAS PubMed.
- (a) M. Wang, M. Li, S. Yang, X.-S. Xue, X. Wu and C. Zhu, Radical-mediated C-C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity, Nat. Commun., 2020, 11, 672–678 CrossRef PubMed; (b) H. M. Ko and G. Dong, Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4+2] coupling, Nat. Chem., 2014, 6, 739–744 CrossRef CAS PubMed; (c) Y. Xia, G. Lu, P. Liu and G. Dong, Catalytic activation of carbon–carbon bonds in cyclopentanones, Nature, 2016, 539, 546–550 CrossRef CAS PubMed; (d) Y. Xia, J. B. Wang and G. Dong, Distal-Bond-Selective C−C activation of ring-fused cyclopentanones: an efficient access to spiroindanones, Angew. Chem., Int. Ed., 2017, 56, 2376–2380 CrossRef CAS PubMed; (e) Y. Xia, J. B. Wang and G. Dong, Suzuki–Miyaura coupling of simple ketones via activation of unstrained carbon–carbon bonds, J. Am. Chem. Soc., 2018, 140, 5347–5351 CrossRef CAS PubMed.
- Y. Xu, X. Qi, P. Zheng, C. C. Berti, P. Liu and G. Dong, Deacylative transformations of ketones via aromatization-promoted C–C bond activation, Nature, 2019, 567, 373–378 CrossRef CAS PubMed.
- M. Tian, X. Shi, X. Zhang and X. Fan, Synthesis of 4-acylpyrazoles from saturated ketones and hydrazones featured with multiple C(sp3)–H bond functionalization and C–C bond cleavage and reorganization, J. Org. Chem., 2017, 82, 7363–7372 CrossRef CAS PubMed.
- For representative works on ring-opening of strained cycloalkanamines, see: (a) S. Maity, M. Zhu, R. S. Shinabery and N. Zheng, Intermolecular [3+2] cycloaddition of cyclopropylamines with olefins by visible-light photocatalysis, Angew. Chem., Int. Ed., 2012, 51, 222–226 CrossRef CAS PubMed; (b) Y. Cai, J. Wang, Y. Zhang, Z. Li, D. Hu, N. Zheng and H. Chen, Detection of fleeting amine radical cations and elucidation of chain processes in visible-light-mediated [3+2] annulation by online mass spectrometric techniques, J. Am. Chem. Soc., 2017, 139, 12259–12266 CrossRef CAS PubMed; (c) J. Wang and N. Zheng, The Cleavage of a C-C bond in cyclobutylanilines by visible-light photoredox catalysis: development of a [4+2] annulation method, Angew. Chem., Int. Ed., 2015, 54, 11424–11427 CrossRef CAS PubMed; (d) S. A. Morris, J. Wang and N. Zheng, The prowess of photogenerated amine radical cations in cascade reactions: from carbocycles to heterocycles, Acc. Chem. Res., 2016, 49, 1957–1968 CrossRef CAS PubMed; (e) M.-M. Wang and J. Waser, 1,3-difunctionalization of aminocyclopropanes via dielectrophilic intermediates, Angew. Chem., Int. Ed., 2019, 58, 13880–13884 CrossRef CAS PubMed.
- (a) J. H. Horner, F. N. Martinez, O. M. Musa, M. Newcomb and H. E. Shahin, Kinetics of dialkylaminium cation radical reactions: radical clocks, solvent effects, acidity constants, and rate constants for reactions with hydrogen atom donors, J. Am. Chem. Soc., 1995, 117, 11124–11133 CrossRef CAS; (b) O. M. Musa, J. H. Horner, H. Shahin and M. Newcomb, A kinetic scale for dialkylaminyl radical reactions, J. Am. Chem. Soc., 1996, 118, 3862–3868 CrossRef CAS; (c) W. R. Bowman, C. F. Bridge and P. Brookes, Radical cyclisation onto nitriles, Tetrahedron Lett., 2000, 41, 8989–8994 CrossRef CAS; (d) J. Jin and M. Newcomb, Rate constants and arrhenius functions for ring opening of a cyclobutylcarbinyl radical clock and for hydrogen atom transfer from the Et3B−t3Bical cloc, J. Org. Chem., 2008, 73, 4740–4742 CrossRef CAS PubMed.
- J.-W. Zhang, Y.-R. Wang, J.-H. Pan, Y.-H. He, W. Yu and B. Han, Deconstructive oxygenation of unstrained cycloalkanamines, Angew. Chem., 2020, 59, 3900–3904 CrossRef CAS PubMed.
- For the representative works on N-containing heteroaryl migration via a cyclic amino radical intermediate, see: (a) X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Tertiary-alcohol-directed `functionalization of remote C(sp3)-H bonds by sequential hydrogen atom and heteroaryl migrations, Angew. Chem., Int. Ed., 2018, 57, 1640–1644 CrossRef CAS PubMed; (b) J. Yu, Z. Wu and C. Zhu, Efficient docking–migration strategy for selective radical difluoromethylation of alkenes, Angew. Chem., Int. Ed., 2018, 57, 17156–17160 CrossRef CAS PubMed; (c) M. Wang, Z. Wu, B. Zhang and C. Zhu, Azidoheteroarylation of unactivated olefins through distal heteroaryl migration, Org. Chem. Front., 2018, 5, 1896–1899 RSC; (d) D. Chen, Z. Wu, Y. Yao and C. Zhu, Phosphinoyl-functionalization of unactivated alkenes through phosphinoyl radical-triggered distal functional group migration, Org. Chem. Front., 2018, 5, 2370–2374 RSC; (e) H. Zhang, X. Wu, Q. Zhao and C. Zhu, Copper-catalyzed heteroarylsilylation of unactivated olefins through distal heteroaryl migration, Chem. - Asian J., 2018, 13, 2453–2457 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2020 |