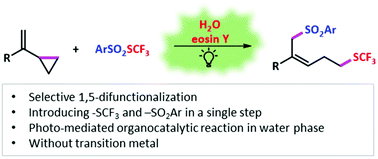
Organocatalytic 1,5-trifluoromethylthio-sulfonylation of vinylcyclopropane mediated by visible light in the water phase†
Abstract
1,2-Difunctionalization has developed into a robust tool for the preparation of complex organic molecules, and remote difunctionalization has also aroused widespread interest to achieve pluripotency of difunctionalization. Herein, we report a metal-free organocatalytic system mediated by visible light to realize the remote 1,5-trifluoromethylthio-sulfonylation of vinylcyclopropane with ArSO2SCF3 reagents in the water phase. This reaction exhibits broad functional group compatibility, moderate to good yields, and high regioselectivities and stereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...