DOI: 10.1039/C9QO90110H
(Profile)
Org. Chem. Front., 2020, 7, 723-725
Interview with Arjan W. Kleij
aInstitute of Chemical Research of Catalonia (ICIQ), the Barcelona Institute of Science & Technology (BIST), Avinguda Països Catalans 16, 43007, Tarragona, Spain. E-mail: akleij@iciq.es; Fax: +34 977 920 823; Tel: +34 977 920 247 Web: http://www.iciq.org/research/research_group/prof-arjan-w-kleij/
bCatalan Institute of Research and Advanced Studies (ICREA), Passeig Lluis Companys 23, 08010, Barcelona, Spain
Received
11th December 2019
, Accepted 11th December 2019
What inspired you to choose science and eventually become a chemist in the first place?
I think it was because of a strong fascination for science fuelled by curiosity, and having experimentation available to discover the “unknown”. In a broader sense and more projected onto chemistry, I always highly appreciated the art of creating new molecules and functions. In this way, I can really see a parallel between contemporary artists and chemists, and the precise control we can exercise as synthetic chemists to design functional molecules.What do you consider to be the main potential areas for future development in the field of small molecule synthesis and conversion?
Well, chemistry in the area of small molecule activation has progressed phenomenally and catalysis is certainly one of the key technologies to valorize such molecules that are sometimes even considered to be waste. I foresee great potential in merging green chemistry concepts (such as the realization of a circular economy) and the organic chemistry of small molecule conversions like for CO2. In addition, small molecule synthesis from waste plastics can also be a highly rewarding and innovative task within the context of organic chemistry. As such, it may alleviate the problems that we are currently facing with plastic pollution and micro-plastics in particular.How do you define ‘a successful scientist’ and suggest to achieve it?
Success in my view is best defined as a discovery that radically changes the way scientists conceive existing paradigms. I always state to younger colleagues that if each scientist could make just one such career discovery, the world would be a much better place. On a personal level, I also get a lot of positive energy from the education of younger people and seeing them growing into mature scientists. Success is not limited to executing science but also includes passing on the knowledge you have gained over the years and hoping that someone will use it for a major discovery at some point in time.What do you think is the most interesting part of your research work?
The most attractive aspect is getting confronted with the unexpected: what is more beautiful than being informed by a grad student or postdoc about a completely new transformation, mechanism or application? Crucial in this respect are the many informal discussions that I have with my team, and the ideas that are generated because of it, thereby pushing the boundaries of our imagination.How does being a scientist allow you to explore other interests in life?
As a scientist, I have a certain liberty to use part of my time in a flexible way and thus to organize my day/week how I see fit. This means I can participate in other interesting events, such as joining as an expert a debate competition among high school students on the use of plastics, and spend time with my family at times typically reserved for work.What are your suggestions for the younger generation to encourage them to consider a career in science?
I think a career in science offers a way to merge creativity and a solution-orientation mind and offers the possibility to work together with other bright minds to overcome multifaceted challenges we are dealing with now and will face in the future. Additionally, if you like to discuss and apply science both theoretically and practically, then a future as scientist is more than worth the investment.How do you feel about your role as an Associate Editor of Organic Chemistry Frontiers?
I feel really excited to be part of a fantastic team of professionals allowing me to be able to contribute to the success of this ambitious journal. While being one of the Associate Editors, I feel that I can truly use my experience to attain the high quality publishing standards, and of course contribute to a further growth of the journal.References
- L. Hu, A. Cai, Z. Wu, A. W. Kleij and G. Huang, A Mechanistic Analysis of the Pd-Catalyzed Formation of Branched Allylic Amines reveals the Origin of the Regio- and Enantioselectivity through a Unique Inner-Sphere Pathway, Angew. Chem., Int. Ed., 2019, 58, 14694–14702 Search PubMed.
- R. Huang, J. Rintjema, J. González Fabra, E. Martín, E. C. Escudero-Adán, C. Bo, A. Urakawa and A. W. Kleij, Deciphering Key Intermediates in the Transformation of Carbon Dioxide into Heterocyclic Products, Nat. Catal., 2019, 2, 62–70 Search PubMed.
- S. Sopeña, M. Cozzolino, C. Maquilón, M. Martínez Belmonte, E. C. Escudero-Adán and A. W. Kleij, Organocatalyzed Domino [3 + 2] Cycloaddition/Payne-Type Rearrangement using Carbon Dioxide and Epoxy Alcohols, Angew. Chem., Int. Ed., 2018, 57, 11203–11207 Search PubMed.
- N. Kindermann, A. Cristòfol and A. W. Kleij, Access to Biorenewable Polycarbonates with Unusual Glass-Transition Temperature (Tg) Modulation, ACS Catal., 2017, 7, 3860–3863 Search PubMed.
- A. Cai, W. Guo, L. Martínez-Rodríguez and A. W. Kleij, Palladium-Catalyzed Regio- and Enantio-Selective Synthesis of Allylic Amines Featuring Tetrasubstituted Tertiary Carbons, J. Am. Chem. Soc., 2016, 138, 14194–14197 Search PubMed.
- J. Rintjema, R. Epping, G. Fiorani, E. Martín, E. C. Escudero-Adán and A. W. Kleij, Substrate Controlled Product Divergence in CO2 Conversion to Heterocyclic Products, Angew. Chem., Int. Ed., 2016, 55, 3972–3976 Search PubMed.
- J. Rintjema, W. Guo, E. Martin, E. C. Escudero-Adán and A. W. Kleij, Highly Chemo-Selective Catalytic Coupling of Substituted Oxetanes and Carbon Dioxide, Chem. – Eur. J., 2015, 21, 10754–10762 Search PubMed.
- V. Laserna, G. Fiorani, C. J. Whiteoak, E. Martin, E. Escudero-Adán and A. W. Kleij, Carbon Dioxide as a Protecting Group: Highly Efficient and Selective Catalytic Access to Cyclic cis-Diol Scaffolds, Angew. Chem., Int. Ed., 2014, 53, 10416–10419 Search PubMed.
- M. V. Escárcega-Bobadilla, G. A. Zelada-Guillén, S. V. Pyrlin, M. Wegrzyn, M. M. D. Ramos, E. Giménez, A. Stewart, G. Maier and A. W. Kleij, Nanorings and Rods Interconnected by Self-Assembly Mimicking an Artificial Network of Neurons, Nat. Commun., 2013, 4, 2648 Search PubMed.
- C. J. Whiteoak, N. Kielland, V. Laserna, E. C. Escudero-Adán, E. Martin and A. W. Kleij, A Powerful Aluminum Catalyst for the Synthesis of Highly Functional Organic Carbonates, J. Am. Chem. Soc., 2013, 135, 1228–1231 Search PubMed.
| This journal is © the Partner Organisations 2020 |


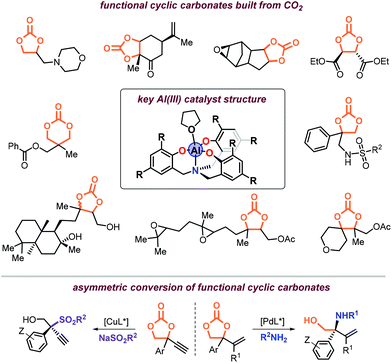
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. His current h-index is around 56. He has been a guest editor for various journals including Catalysis Science & Technology and Advanced Synthesis & Catalysis, and recently joined Organic Chemistry Frontiers as an Associate Editor. He is the chair of the Carbon Dioxide Conversion Catalysis (CDCC) conference series, and in 2019 he also chaired the 4th EuCheMS Congress on Green and Sustainable Chemistry in Tarragona. His main research interests are in the area of small molecule valorization catalysis, development of new reactivity using organic carbonates as modular scaffolds, and the use of renewable compounds and monomers in stereo-selective transformations
000. His current h-index is around 56. He has been a guest editor for various journals including Catalysis Science & Technology and Advanced Synthesis & Catalysis, and recently joined Organic Chemistry Frontiers as an Associate Editor. He is the chair of the Carbon Dioxide Conversion Catalysis (CDCC) conference series, and in 2019 he also chaired the 4th EuCheMS Congress on Green and Sustainable Chemistry in Tarragona. His main research interests are in the area of small molecule valorization catalysis, development of new reactivity using organic carbonates as modular scaffolds, and the use of renewable compounds and monomers in stereo-selective transformations