Efficient labeling of organic molecules using 13C elemental carbon: universal access to 13C2-labeled synthetic building blocks, polymers and pharmaceuticals†
Abstract
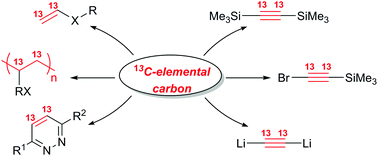
Among different types of labeling, 13C-labeled compounds are the most demanding in organic chemistry, life sciences and materials design. However, 13C-labeled organic molecules are very difficult to employ in practice due to extreme cost. The rather narrow range of labeled organic starting materials and the absence of universal synthetic building units further complicate the problem and make utilization of 13C-labeled molecules hardly possible in many cases. Here we report a versatile approach for 13C2-labeling of organic molecules starting with 13C elemental carbon: 13C carbon is applied for the synthesis of calcium carbide (Ca13C2), which is subsequently used to generate acetylene – a universal 13C2 unit for atom-economic organic transformations. Syntheses of labeled alkynes, O,S,N-functionalized vinyl derivatives, polymers and pharmaceutical substances were demonstrated. Elemental 13C carbon, as the chemically most simple source for 13C2-labeling, here was successfully combined with universal synthetic applicability of alkynes.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...