Rapid, ultrasensitive and highly specific biosensor for the diagnosis of SARS-CoV-2 in clinical blood samples
Abstract
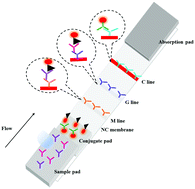
In this study, a lateral flow combined IgG–IgM immunochromatographic assay is developed for the rapid, simultaneous detection of IgM and IgG antibodies against SARS-CoV-2 in clinical blood samples within 15 min. The clinical detection sensitivity and specificity of the assay strips is investigated in samples of blood from inpatients with COVID-19. The sensitivity and specificity of this assay are 85.29% and 100.00%, respectively. Compared with a single IgG and IgM test, the combined IgG–IgM immunochromatographic strip test has higher sensitivity. Our results demonstrate that the combined IgG–IgM immunochromatographic strip is suitable for the rapid screening of SARS-CoV-2 infection, among confirmed COVID-19 patients, suspect patients and asymptomatic SARS-CoV-2 carriers.
- This article is part of the themed collection: 2020 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...