Protein-assisted synthesis of nanoscale covalent organic frameworks for phototherapy of cancer†
Abstract
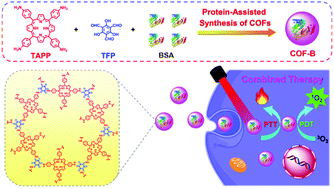
Covalent organic frameworks (COFs) represent a promising class of crystalline porous materials in various fields, but their applications as biomaterials are usually limited by the large sizes and low colloid stability in aqueous solution. Inspired by the biomimetic mineralization strategies for preparing nanoparticles, herein a homogeneous and stable porphyrin-based COF material (COF-B) has been developed with the assistance of bovine serum albumin (BSA), which exhibits both photothermal and photodynamic activity under the excitation of a laser at a single wavelength. COF-B possesses not only good water dispersibility, but also robust photostability and biocompatibility. Moreover, COF-B shows excellent phototherapy efficiency with effective inhibition or even complete elimination of tumors upon irradiation. This protein-assisted synthesis of COF materials propels the preparation of stable nanoscale COFs and the application of COFs as highly efficient biomedical materials.
- This article is part of the themed collection: 2020 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...