Oxygen vacancy induced superior visible-light-driven photo-catalytic performance in the BiOCl homojunction
Abstract
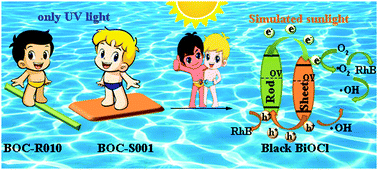
The photocatalytic performance of semiconductors can be enhanced by expanding the spectral response range and accelerating the photo-induced charge separation. Introduction of oxygen vacancies and construction of homo-junctions in BiOCl were adopted to widen the absorption spectra and reduce the photogenerated electron–hole pair recombination, further enhancing its photocatalytic activity. Black BiOCl with oxygen vacancies and a homo-junction was fabricated using a simple one-pot hydrothermal method. Meanwhile, white BiOCl nanosheets and nanorods with crystal growth in different orientations were simultaneously synthesized to compare with the black BiOCl homo-junction, which exposed the same crystal facets as those of black BiOCl. High resolution transmission electron microscopy (HRTEM) was performed to confirm the formation of the crystal-facet homo-junction, while the existence of oxygen vacancies in black BiOCl was proved by X-ray photoelectron spectroscopy (XPS) and electron spin resonance (ESR) spectroscopy. The spectral absorption range and the photo-generated electron–hole pair separation ability of catalysts were characterized by UV-vis diffuse reflectance absorption spectroscopy (UV-vis DRS), transient photocurrent density and electrochemical impedance spectroscopy (EIS). The photo-catalytic activities of the black BiOCl homo-junctions were evaluated through degradation of RhB and disinfection performance towards E. coli and S. aureus, and they can degrade 99.9% of RhB solution and disinfect 97% of bacteria under simulated sunlight irradiation. Further, the possible photo-catalytic mechanism and the improvement of photo-catalytic performance could be ascribed to the introduction of oxygen vacancies and the construction of homo-junctions, which was also confirmed by theoretical computation.
- This article is part of the themed collection: 2020 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...