Ligand exchange on noble metal nanocrystals assisted by coating and etching of cuprous oxide†
Abstract
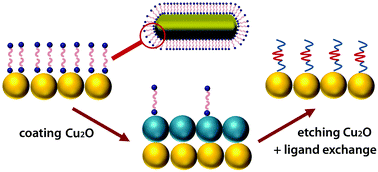
We report an oxide-assisted coating-etching process to remove bio-incompatible capping ligands from the surface of noble metal nanocrystals. The method involves the growth of a layer of cuprous oxide (Cu2O) on the nanocrystal surface to compete with the existing ligands, followed by selective etching of the Cu2O layer in the presence of a new ligand. Such a ligand exchange process has its significance in the biomedical applications of noble metal nanocrystals as many of them are not biocompatible due to the cytotoxicity of the original capping ligands. We demonstrate the efficacy of this strategy by focusing on cetyltrimethylammonium bromide-capped gold (CTAB-Au) nanorods, a class of very useful plasmonic nanomaterials with well-known bio-incompatibility due to the cytotoxicity of CTAB. After coating and etching of Cu2O on AuNRs, the CTAB ligands on the nanocrystal surface can be readily replaced by a poloxamer ligand F127, and the resulting AuNRs can be used in computed tomography and optical coherence tomography imaging with higher contrast enhancement than those capped with CTAB ligands. This strategy is scalable, general, and extendable to other types of nanocrystals, and it is expected to open up many new opportunities that have been not possible previously due to the bio-incompatibility of the nanomaterial.
- This article is part of the themed collection: 2020 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...