Open Access Article
Open Access ArticleExpanding the tunability and applicability of exchange-coupled/decoupled magnetic nanocomposites†
Cecilia
Granados-Miralles
 *a,
Adrián
Quesada
*a,
Adrián
Quesada
 a,
Matilde
Saura-Múzquiz‡
a,
Matilde
Saura-Múzquiz‡
 b,
Henrik L.
Andersen§
b,
Henrik L.
Andersen§
 b,
José F.
Fernández
b,
José F.
Fernández
 a and
Mogens
Christensen
a and
Mogens
Christensen
 b
b
aElectroceramics Department, Instituto de Cerámica y Vidrio, CSIC, Kelsen 5, ES 28049 Madrid, Spain. E-mail: c.granados.miralles@icv.csic.es
bCenter for Materials Crystallography, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, DK 8000 Aarhus, Denmark
First published on 28th February 2020
Abstract
CoFe2O4/Co–Fe magnetic composites are usually prepared through partial reduction of CoFe2O4, which often yields monoxides (i.e., FeO, CoO) as secondary phases. Since these compounds are paramagnetic at ambient conditions, the presence of a small amount of monoxide is generally downplayed in the literature, and the possible effects on the magnetic properties are simply ignored. However, the present study shows that even a low concentration of monoxide results in decoupling of the soft and hard magnetic phases, which inevitably leads to a deterioration of the magnetic properties. Additionally, it is confirmed that a partial reduction of CoFe2O4 is a suitable method to produce CoFe2O4/Co–Fe nanocomposites, provided that the treatment is well controlled with respect to duration, temperature and flow of reductant. A monoxide-free nanocomposite was produced and its magnetic properties evaluated both at room and low temperature. Our model system exemplifies the potential of exchange-coupling (and decoupling) as a tool to tune the magnetic properties of a material within a relatively wide range of values, thus widening its spectrum of potential applications.
Introduction
Magnetic nanoparticles (MNPs) have undoubtedly been one of the hot research topics of the 21st century.1 Intensive research on the subject has yielded notable advances in a wide range of technologies and disciplines. For instance, MNPs have been a great aid in medical diagnosis and treatment of diseases.2 Among other cutting-edge medical applications, MNPs are integral components of drug carriers for magnetic drug delivery,3,4 heat mediators in cancer therapy by magnetic fluid hyperthermia (MFH),5 or contrast agents for magnetic resonance imaging (MRI).6 MNPs are also highly relevant in matter of sensors and biosensors aimed to diverse analytes,7e.g., food contaminants,8,9 environmental pollutants,10 antibodies,11etc.The actual application determines the required magnetic properties. Very often, the stability and longevity of the devices rely on a strong resistance to demagnetization (i.e. hard magnetic material, with large coercivity, Hc). Other times, the crucial parameter that ensures compliance with the specific task is the ability of the material to become magnetized up to a high value (i.e. high saturation magnetization, Ms). Most of the available materials show either a large Hc and a moderate Ms or vice versa.12 Consequently, if relatively high values of both Hc and Ms are necessary, fabrication of composite materials should be addressed. According to the exchange-spring theory, the Ms of a hard magnetic material can be enhanced by adding a controlled amount of a large-Ms material (generally soft), and the cost in Hc will be low provided that the two materials are effectively exchange-coupled.13
Ferrites are among the most used magnetic materials, owing to their good magnetic properties, chemical and mechanical stability, and the availability of elements they are based on. Especially interesting are the spinel ferrites (SFs), as they allow easy tunability of the magnetic properties with small changes on the chemical composition,14–16 thus increasing their versatility towards different applications. SFs have been widely used in the electronic industry, for high-density data storage and spintronic devices.17,18 Their utilization for biomedical applications has increased significantly over the last years, especially in the fields of drug delivery19 and biosensors.20,21 In addition to their applications as magnetic materials, it is worth mentioning that SFs are widely used for other purposes, e.g., as catalysts for very varied chemical processes,22,23 advanced battery electrodes,24,25 electrochemical supercapacitors in energy storage systems,26etc.
SFs have the general formula M2+(Fe3+)2O4, with M = Mg, Mn, Fe, Co, Ni, Cu, Zn.17 Out of all them, only Co-spinel shows hard magnetic properties, while the rest are soft magnetic species.27 Moreover, CoFe2O4 can be easily reduced to a Co–Fe alloy in the presence of a small concentration of H2 gas and moderate temperatures (≈300 °C).28 Both facts make this compound interesting, as an incomplete CoFe2O4 reduction directly leads to coexistence of hard (CoFe2O4) and soft (Co–Fe) magnetic phases. This is an excellent tool from the material science viewpoint, as it offers the potential to fine tuning the soft/hard magnetic behavior of the produced material by means of controlling the composite composition.
For the above reasons, numerous studies on the CoFe2O4 (hard)/Co–Fe (soft) composite are found in the literature, including composites prepared as powders,29 dense pellets,30 or thin films.31 Some works have set the main focus on the preparation process (in situ studies),28,32 while others have taken care of an in-depth structural characterization of the produced composites using spectroscopic techniques such as Raman33 or Mössbauer spectroscopy.34,35 Others have put great efforts on studying the inter-particle coupling from different perspectives, both using transmission electron microscopy (TEM), and measuring δm curves (Henkel plots).35,36 Recently, micromagnetic calculations on these systems have also been reported.37 However, a successful exchange-coupling of these two magnetic phases has proven rather challenging to achieve, the reason behind it often remaining unclear. In the present work, the origin of magnetic decoupling in CoFe2O4/Co–Fe nanocomposites is addressed. Composites covering a range of compositions are prepared, and their crystalline and atomic structures are studied using high-resolution powder X-ray diffraction. Physical characterization of the magnetic properties is carried out both at room and low temperature, and coupling/decoupling of the system is evaluated in terms of the phases present in the sample and their average crystallite sizes.
Experimental
Sample preparation
Magnetic CoFe2O4/Co–Fe nanocomposites were prepared by means of a controlled reduction of CoFe2O4 nanoparticles. The starting CoFe2O4 material was hydrothermally synthesized following the procedure described in a previous work,38 and had a volume-averaged crystallite size of 14.4(1) nm. 0.20 g of the as-synthesized powders were spread on an Al2O3 crucible with approximate dimensions 60 × 40 mm2. The crucible was placed at the hot spot of a tubular furnace (C.H.E.S.A. Ovens). The furnace was sealed at both ends and purged down to a pressure of ≈1 × 10−2 mbar using a vacuum pump connected to the furnace outlet. Gas mixture 10% H2/90% N2 was fed through the furnace inlet, regulating the flow until the pressure inside the furnace stabilized at 20 mbar. Finally, the thermal treatment was initiated. An initial heating ramp of 100 °C min−1 drove the temperature up to the set point (300–600 °C), at which the system was maintained during 2–8 hours (see heating profiles in Fig. S1, ESI†). Subsequently, the sample was left to cool down inside the furnace, while maintaining the flow of reducing gas. The sample was removed from the furnace once the temperature was below 75 °C. All samples were stable in air.Characterization
Rietveld analysis of the PXRD data was performed using the FullProf Suite.39 In the Rietveld model, the oxides were described assuming a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe stoichiometry of 1
Fe stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (i.e., CoFe2O4, Co0.33Fe0.67O) and a random distribution of the two cations among the equivalent crystallographic sites. The elemental composition of the alloy in the model varied depending on the sample. A detailed crystallographic description of all the Rietveld phases may be found on Tables S1–S5 in the ESI.†
2 (i.e., CoFe2O4, Co0.33Fe0.67O) and a random distribution of the two cations among the equivalent crystallographic sites. The elemental composition of the alloy in the model varied depending on the sample. A detailed crystallographic description of all the Rietveld phases may be found on Tables S1–S5 in the ESI.†
Data were also collected on a NIST 660B LaB6 calibrant in the different experimental configurations, and these data were modelled (LeBail fit) to estimate the instrumental contribution to the peak broadening. The instrument contribution was deconvoluted from the samples data, and the remaining profile broadening, originating from the sample, was modelled as Lorentzian isotropic size-broadening using the Thompson–Cox–Hastings formulation of the pseudo-Voigt function.40
Prior to the measurements described above, the room temperature magnetization of the samples was measured in a smaller field range ±4 kOe (318 kA m−1) using a home-built VSM setup.41
Results and discussion
Composition and crystallite size from Rietveld analysis
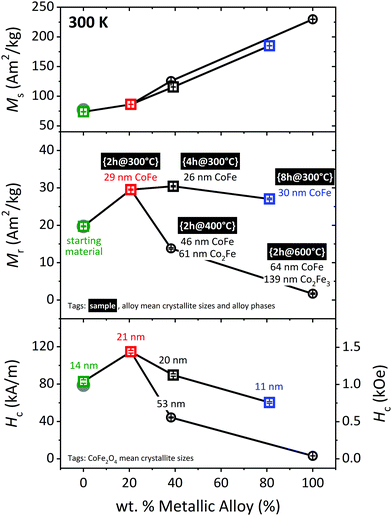
Reduction treatments of variable duration and temperature yielded five different samples. Henceforth, tags in the form {time@temperature} are used to refer to the samples. Sample composition and sizes obtained from Rietveld analysis of the PXRD data collected on those samples are displayed in Fig. 1 and Table 1. A representative example of a Rietveld model fitted to the PXRD data is shown in Fig. 2(a). The Rietveld models fitted to the PXRD data collected for the remaining samples may be found on Fig. S5 in the ESI.†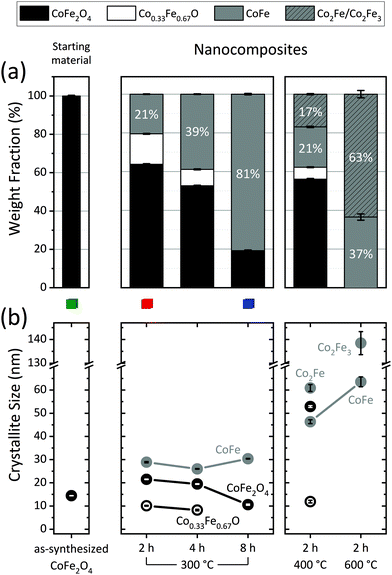 | ||
| Fig. 1 (a) Sample composition and (b) crystallite size of the constituent phases extracted from Rietveld analysis of the PXRD data measured on the starting material and the five different nanocomposites. The magnetic properties of the samples highlighted with a green/red/blue square are represented in Fig. 4 and 5 using the same color-code. | ||
| Sample | CoFe2O4 | Co0.33Fe0.67O | Metallic alloy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 〈D〉 (nm) | a (Å) | wt% | 〈D〉 (nm) | a (Å) | wt% | 〈D〉 (nm) | a (Å) | |||
| Starting material | 100.0(3) | 14.4(1) | 8.3929(1) | — | — | — | — | — | — | — | |
| {2h@300°C} | 63.8(3) | 21.5(1) | 8.3889(1) | 15.7(2) | 10.1(2) | 4.2695(2) | 20.5(1) | CoFe | 28.9(3) | 2.85645(4) | |
| {4h@300°C} | 52.7(3) | 19.5(1) | 8.3886(1) | 8.5(2) | 8.3(3) | 4.2680(4) | 38.9(2) | CoFe | 26.0(2) | 2.85877(4) | |
| {8h@300°C} | 19.1(4) | 10.6(3) | 8.3904(5) | — | — | — | 80.9(4) | CoFe | 30.4(2) | 2.86141(4) | |
| {2h@400°C} | 56.1(4) | 52.9(4) | 8.38952(7) | 6.2(2) | 11.9(6) | 4.2860(4) | 37.8(7) | 16.9(3) | Co2Fe | 61(2) | 2.84639(3) |
| 20.8(3) | CoFe | 46.3(8) | 2.85434(3) | ||||||||
| {2h@600°C} | — | — | — | — | — | — | 100(4) | 37(2) | CoFe | 64(2) | 2.85954(6) |
| 64(2) | Co2Fe3 | 139(5) | 2.86405(3) | ||||||||
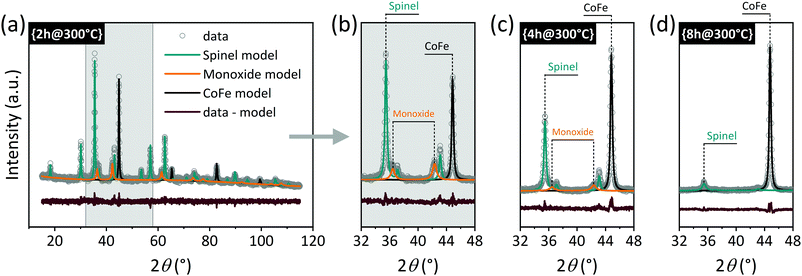 | ||
| Fig. 2 (a) PXRD data and corresponding Rietveld model of the phases present in sample {2h@300°C}. (b) Selected 2θ-region of data and models for {2h@300°C}, (c) {4h@300°C}, and (d) {8h@300°C}. | ||
From the series of experiments at 300 °C with variable duration (2–8 h), it is clear that as time increases, the amount of CoFe2O4 decreases, at the expense of the appearance of reduced phases: a monoxide phase (Co0.33Fe0.67O) and a metallic alloy phase (CoFe). The monoxide seems to play the role of a reaction intermediate, as it disappears as the reduction advances. Thus, while 2 and 4 h at 300 °C produced composites with 16.1(2)% and 8.6(3)% monoxide, respectively, a monoxide-free composite with an 80.9(4)% metallic content was obtained after 8 h. Fig. 2(b–d) show selected 2θ-regions of the PXRD data and models corresponding to these three samples. The distinct Rietveld phases are highlighted to illustrate the appearance/disappearance of the different phases as dwell time increases.
At 300 °C, the growth of the soft phase crystallites remains relatively controlled (≤30.4(2) nm) regardless of the dwell time. Increasing the treatment temperature accelerates the reduction process,28 thus, 2 h at 400 °C led to lower CoFe2O4 content than 2 h at 300 °C. The monoxide content also decreased substantially at 400 °C. At 600 °C, 2 hours were sufficient to completely reduce the starting material to pure metallic phases. However, increasing the temperature entails a significant growth of the alloy crystallites.
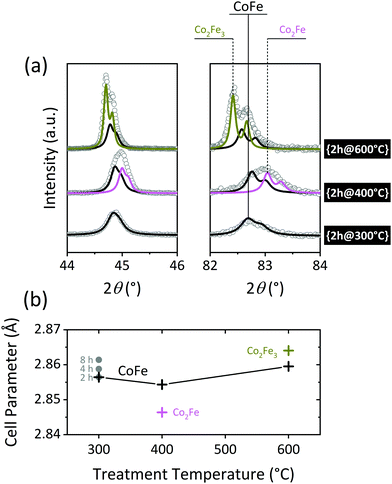
Fig. 3(a) shows the evolution of the most intense reflections of the alloy phase as a function of the reduction temperature. While the diffraction data collected for the {2h@300°C} nanocomposite can be modelled with a single metallic phase (CoFe), at least two metallic phases are clearly present in the {2h@400°C} and {2h@600°C} samples. The refined unit cell parameters for the individual phases are displayed in Table 1 and plotted in Fig. 3(b) as a function of the treatment temperature. The dissimilar distribution of cell parameters suggests different elemental compositions of the alloys. Unfortunately, the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio could not be extracted from the refinements, because Co and Fe are next-neighbors in the periodic table and therefore, practically indistinguishable using X-rays (see ESI in ref. 28).
Fe ratio could not be extracted from the refinements, because Co and Fe are next-neighbors in the periodic table and therefore, practically indistinguishable using X-rays (see ESI in ref. 28).
The unit cell dimensions of Co–Fe alloys increase with an increasing Fe content.42 This allows an estimate of the elemental composition based on the lattice parameter. The empirical chemical compositions shown in Table 1 and Fig. 3 were assessed by substituting the refined unit cell parameters in the equation obtained by Ohnuma et al. for ordered body-centered-cubic (bcc) structures.42 For the mildest reduction, {2h@300°C}, the calculated alloy composition is CoFe. This indicates surplus Co on the alloy, compared to the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe stoichiometry of 1
Fe stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 presumed for the starting spinel material. This observation is in agreement with previous in situ investigations on this system, where the reduced phases were observed to appear in a Co-rich form, to later incorporate Fe and evolve towards Co
2 presumed for the starting spinel material. This observation is in agreement with previous in situ investigations on this system, where the reduced phases were observed to appear in a Co-rich form, to later incorporate Fe and evolve towards Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe = 1
Fe = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.28 At the higher temperatures, CoFe coexists with other alloy phases, i.e., Co2Fe in {2h@400°C} and Co2Fe3 in {2h@600°C}, showing that the Fe-content increases as the temperature rises. A similar phase segregation may be occurring at 300 °C, although the effect remains hidden under the broader diffraction peaks derived from the smaller crystallite sizes at this temperature, and in that case, the refined unit cell parameter should be understood as the weighted average of all the phases present. The cell dimensions increase slightly with dwell time, again indicating a late incorporation of the Fe in the alloy structure.
2.28 At the higher temperatures, CoFe coexists with other alloy phases, i.e., Co2Fe in {2h@400°C} and Co2Fe3 in {2h@600°C}, showing that the Fe-content increases as the temperature rises. A similar phase segregation may be occurring at 300 °C, although the effect remains hidden under the broader diffraction peaks derived from the smaller crystallite sizes at this temperature, and in that case, the refined unit cell parameter should be understood as the weighted average of all the phases present. The cell dimensions increase slightly with dwell time, again indicating a late incorporation of the Fe in the alloy structure.
The influence of the amount of H2 inside the furnace was also investigated (see Fig. S6 in the ESI†). The gas pressure was increased up to 100 and 300 mbar, and no significant changes were observed neither on the sample composition nor the crystallite sizes, compared to the experiments at 20 mbar. This suggests that, for the amounts of sample used here, an H2 excess is ensured even at the lowest pressure, and as long as there is enough H2 available, the gas pressure does not seem to have a major influence on the process.
To evaluate whether the crystallite size of the starting material plays a role, an additional time series of experiments were carried out at 300 °C using CoFe2O4 powders with an average size of 8.2(1) nm (see Fig. S7 in the ESI†). Comparing these results with those represented in Fig. 1 (mean size starting material 14.4(1) nm), it is concluded that the smaller the size of the starting CoFe2O4, the faster the reduction occurs, i.e., the shorter the time required to achieve a certain reduction stage.
Magnetic properties
| Sample | M s (A m2 kg−1) | M r (A m2 kg−1) | H c (kA m−1)a | H c (kOe)a |
|---|---|---|---|---|
| a H c is given both in SI an CGS units to ease comparison with other works. | ||||
| Starting material | 73.9(4) | 19.7(1) | 83(2) | 1.04(2) |
| {2h@300°C} | 86.3(1) | 29.5(1) | 115(1) | 1.44(2) |
| {4h@300°C} | 115.6(1) | 30.4(2) | 90(1) | 1.13(2) |
| {8h@300°C} | 185.1(1) | 27.0(2) | 60.4(9) | 0.76(1) |
| {2h@400°C} | 125.6(1) | 13.8(2) | 44.3(6) | 0.557(7) |
| {2h@600°C} | 229.7(2) | 1.7(2) | 3.23(2) | 0.0406(2) |
In order to discriminate the influence of the temperature from the effect of the actual reduction process, a 2 h long treatment in vacuum at 400 °C was carried out. No significant changes were observed in the magnetic properties after this treatment (see solid, gray circles in Fig. 4). Therefore, in the following, the starting CoFe2O4 powders will continue to be used as reference to evaluate the magnetic properties of the nanocomposites.
M s follows the expected linear increase with the amount of alloy present in the sample. The trends exhibited by Mr and Hc are slightly more complex. A mild reduction, such as {2h@300°C} (in red color) yields a significant enhancement of both parameters; the composite with a 20.5(1) wt% alloy has a 50% higher Mr and a 39% larger Hc than the starting material. This is understood as a consequence of the temperature which causes a moderate growth of the CoFe2O4 nanoparticles, from 14.4(1) to 21.5(1) nm, and has very likely induced a betterment of the crystallinity as well.
As the alloy wt% increases, both Mr and Hc decrease, but the decrease is much more pronounced for the temperature series (circles) than for the time series (squares). For instance, the {4h@300°C} nanocomposite has a Mr = 30.4(2) A m2 kg−1 and a Hc = 90(1) kA m−1, and these parameters are reduced by more than half for the sample with approximately the same composition fabricated at 400 °C for 2 h (Mr = 13.8(2) A m2 kg−1, Hc = 44.3(6) kA m−1). Despite the similarity in composition between these two samples, the crystallite sizes of both hard and soft phases are much larger for the composite prepared at the higher temperature, which can explain the deterioration of the magnetic properties: (i) the 52.9(4) nm refined for the hard phase in {2h@400°C} is above the critical stable single-domain size (SSD) for CoFe2O4 (≈40 nm),44 which explains the collapse in Hc observed for this sample. (ii) The alloy also grows well beyond typical SSD values, and formation of domains in the soft phase eases spontaneous demagnetization of the hard when both phases are coupled.31
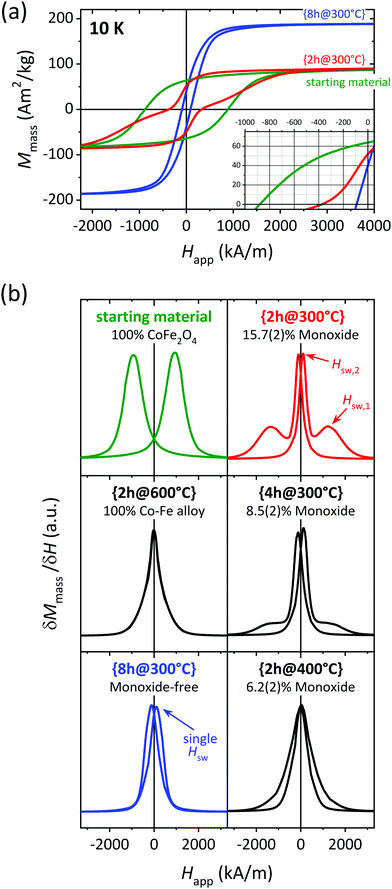 | ||
| Fig. 5 (a) Low temperature magnetic hysteresis loops for selected samples and (b) corresponding first derivative normalized. The samples represented in green, red and blue color in this figure are highlighted with the same colors in Fig. 1 and 4. | ||
The number of reversal or switching events is readily revealed by the maxima in the first derivative curve of the magnetization data. First derivatives of the M–H data from all samples are displayed in Fig. 5(b). The starting material shows the single-step behavior expected for a pure phase, with a single switching field, Hsw, at ≈940 kA m−1. The same is observed for the fully-reduced sample {2h@600°C} but with a nearly zero Hsw. Note the shape of the peaks here is much more Lorentzian than for the starting material. This shape can result from the convolution of several independent contributions from distinct phases (rather than a single-phase), all of them having a very similar, nearly-null magnetic anisotropy. This is in agreement with the two bcc species with different Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios visible in the PXRD data.
Fe ratios visible in the PXRD data.
Two very distinct Hsw are detected for {2h@300°C} (red), which is an indicative of weakly exchanged soft–hard interphases. On the contrary, {8h@300°C} (blue) presents a single-step reversal, which in this case is attributed to an effective of exchange-coupling between the soft and hard phases. Independent magnetization reversal of the magnetic phases is visible for {4h@300°C}, although the peak defined by the larger Hsw is much less intense compared to the 2 h experiment at the same temperature (red curve). The δM/δH curve for {2h@400°C} is maximized at a single Hsw value. However, the peaks here are not symmetric and the peak tails do not coincide, suggesting some degree of decoupling of the two magnetic phases.
To summarize, the only composite showing LT exchange-coupling behavior is the monoxide-free sample {8h@300°C} (blue color). We believe this observation is far from coincidental, considering the correlation between the monoxide concentration and the degree of decoupling shown by our data (see plots on the right from Fig. 5(b)). The present study demonstrates how avoiding the monoxide is imperative for producing effectively exchange-coupled CoFe2O4/Co–Fe nanocomposites. This observation is consistent with and may help explain previous literature on the subject. Several studies report decoupling at RT in monoxide-containing samples29,45–48 Some decoupled “monoxide-free” examples are also found.49–53 However, we consider it possible that the monoxide was overlooked in those works. The proximity in 2θ of the monoxide and the spinel Bragg positions (see Fig. 2) makes it difficult to separate the contribution from these two phases unless the PXRD data has high enough resolution and the subsequent data analysis is appropriate.
Based on a joint interpretation of the RT and LT magnetization data, we understand that our monoxide-free, exchange-coupled sample {8h@300°C} is far from reaching the best magnetic properties the CoFe2O4/Co–Fe system allows. Samples with a lower alloy content, such as {2h@300°C} and {4h@300°C}, appear significantly more promising, owing to their higher Mr and Hc values at RT (see Fig. 4), despite the presence of monoxide hindering the optimal magnetic performance of these samples. Therefore, we believe that monoxide-free composites with an alloy content ≲40 wt% may lead to effectively exchange-coupled CoFe2O4/Co–Fe composites with superior magnetic properties, and should thus be pursued in future work.
Conclusions
CoFe2O4 (hard)/Co–Fe (soft) magnetic nanocomposites have been prepared through controlled partial reduction of CoFe2O4 nanoparticles, obtaining samples with several compositions and crystallite sizes. An additional monoxide phase was found in some of the samples, although this phase disappeared for long reaction times. Magnetization curves at room and low temperature reveal that an increasing monoxide concentration deteriorates inter-phase magnetic exchange-coupling. In fact, the only composite showing an effective exchange-coupling was monoxide-free. Thus, minimizing/avoiding the formation of the monoxide is crucial for producing effectively exchange-coupled CoFe2O4/Co–Fe nanocomposites.Once the chemistry behind the process is understood, partial reduction of CoFe2O4 is a very strong method for synthesizing CoFe2O4/Co–Fe nanocomposites with controlled magnetic properties. Adjusting each of the reduction parameters (temperature, time, partial H2 pressure, crystallite size of the starting CoFe2O4 powders) has a very specific impact on the composition and crystallite sizes of the obtained nanocomposite, which, in turn, directly determines its magnetic behavior. The present work reveals exchange-coupling to be an excellent tool to further expand the range within which the magnetic properties of spinel ferrites can be tuned, extending the scope of this family of compounds. The method described here using CoFe2O4/Co–Fe as an example may in principle be applicable to other ferrite systems, including hard hexaferrites or other spinel ferrites (soft), and allows multiple combinations of magnetic compounds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. G.-M. and A. Q. have contributed equally to this work. The authors would like to thank financial support from the European Commission through the AMPHIBIAN project (H2020-NMBP-2016-720853), the Danish National Research Foundation (Center for Materials Crystallography, DNRF-93), and the Spanish Ministerio de Ciencia, Innovación y Universidades (RTI2018-095303-A-C52). C. G.-M. acknowledges financial support from the Spanish Ministerio de Ciencia, Innovación y Universidades through the Juan de la Cierva Program (FJC2018-035532-I). Authors from Aarhus University gratefully acknowledge affiliation with the Center for Integrated Materials Research (iMAT) at Aarhus University. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).Notes and references
- D. L. Andrews, R. H. Lipson and T. Nann, Comprehensive Nanoscience and Nanotechnology, Elsevier B.V., 2019 Search PubMed.
- H. M. Williams, The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases, Biosci. Horizons Int. J. Student Res., 2017, 10, 1–10 CAS.
- Y.-L. Liu, D. Chen, P. Shang and D.-C. Yin, A review of magnet systems for targeted drug delivery, J. Controlled Release, 2019, 302, 90–104 CrossRef CAS PubMed.
- P. Dong, K. P. Rakesh, H. M. Manukumar, Y. H. E. Mohammed, C. S. Karthik, S. Sumathi, P. Mallu and H. L. Qin, Innovative nano-carriers in anticancer drug delivery-a comprehensive review, Bioorg. Chem., 2019, 85, 325–336 CrossRef CAS PubMed.
- D. Chang, M. Lim, J. A. C. M. Goos, R. Qiao, Y. Y. Ng, F. M. Mansfeld, M. Jackson, T. P. Davis and M. Kavallaris, Biologically Targeted Magnetic Hyperthermia: Potential and Limitations, Front. Pharmacol., 2018, 9, 831 CrossRef.
- K. Wu, D. Su, R. Saha, D. Wong and J.-P. Wang, Magnetic particle spectroscopy-based bioassays: methods, applications, advances, and future opportunities, J. Phys. D: Appl. Phys., 2019, 52, 173001 CrossRef CAS.
- T. A. P. Rocha-Santos, Sensors and biosensors based on magnetic nanoparticles, TrAC, Trends Anal. Chem., 2014, 62, 28–36 CrossRef CAS.
- G. Bülbül, A. Hayat and S. Andreescu, Portable Nanoparticle-Based Sensors for Food Safety Assessment, Sensors, 2015, 15, 30736–30758 CrossRef PubMed.
- Y. Li, Z. Wang, L. Sun, L. Liu, C. Xu and H. Kuang, Nanoparticle-based sensors for food contaminants, TrAC, Trends Anal. Chem., 2019, 113, 74–83 CrossRef CAS.
- D. Song, R. Yang, F. Long and A. Zhu, Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants, J. Environ. Sci., 2019, 80, 14–34 CrossRef PubMed.
- M. Pastucha, Z. Farka, K. Lacina, Z. Mikušová and P. Skládal, Magnetic nanoparticles for smart electrochemical immunoassays: a review on recent developments, Microchim. Acta, 2019, 186, 312 CrossRef PubMed.
- J. M. Soares, V. B. Galdino and F. L. A. Machado, Exchange-bias and exchange-spring coupling in magnetic core–shell nanoparticles, J. Magn. Magn. Mater., 2014, 350, 69–72 CrossRef CAS.
- E. F. Kneller and R. Hawig, The exchange-spring magnet: a new material principle for permanent magnets, IEEE Trans. Magn., 1991, 27, 3588–3600 CAS.
- G. Muscas, N. Yaacoub, G. Concas, F. Sayed, R. Sayed Hassan, J. M. Greneche, C. Cannas, A. Musinu, V. Foglietti, S. Casciardi, C. Sangregorio and D. Peddis, Evolution of the magnetic structure with chemical composition in spinel iron oxide nanoparticles, Nanoscale, 2015, 7, 13576–13585 RSC.
- H. L. Andersen, M. Saura-Múzquiz, C. Granados-Miralles, E. Canévet, N. Lock and M. Christensen, Crystalline and magnetic structure–property relationship in spinel ferrite nanoparticles, Nanoscale, 2018, 10, 14902–14914 RSC.
- H. L. Andersen, C. Granados-Miralles, M. Saura-Múzquiz, M. Stingaciu, J. Larsen, F. Søndergaard-Pedersen, J. V. Ahlburg, L. Keller, C. Frandsen and M. Christensen, Enhanced intrinsic saturation magnetization of ZnxCo1−xFe2O4 nanocrystallites with metastable spinel inversion, Mater. Chem. Front., 2019, 3, 668–679 RSC.
- T. Tatarchuk, M. Bououdina, J. J. Vijaya and L. J. Kennedy, in Nanophysics, Nanomaterials, Interface Studies, and Applications, ed. O. Fesenko and L. Yatsenko, Springer International Publishing, 2017, pp. 305–325 Search PubMed.
- A. Hirohata, H. Sukegawa, H. Yanagihara, I. Zutic, T. Seki, S. Mizukami and R. Swaminathan, Roadmap for Emerging Materials for Spintronic Device Applications, IEEE Trans. Magn., 2015, 51, 1–11 Search PubMed.
- M. Amiri, M. Salavati-Niasari and A. Akbari, Magnetic nanocarriers: Evolution of spinel ferrites for medical applications, Adv. Colloid Interface Sci., 2019, 265, 29–44 CrossRef CAS.
- S. M. Hosseini, S. Sohrabnejad, G. Nabiyouni, E. Jashni, B. Van der Bruggen and A. Ahmadi, Magnetic cation exchange membrane incorporated with cobalt ferrite nanoparticles for chromium ions removal via electrodialysis, J. Membr. Sci., 2019, 583, 292–300 CrossRef CAS.
- Y. Wang, H. Li, L. Guo, Q. Jiang and F. Liu, A cobalt-doped iron oxide nanozyme as a highly active peroxidase for renal tumor catalytic therapy, RSC Adv., 2019, 9, 18815–18822 RSC.
- C. Wei, Z. Feng, M. Baisariyev, L. Yu, L. Zeng, T. Wu, H. Zhao, Y. Huang, M. J. Bedzyk, T. Sritharan and Z. J. Xu, Valence Change Ability and Geometrical Occupation of Substitution Cations Determine the Pseudocapacitance of Spinel Ferrite XFe2O4 (X = Mn, Co, Ni, Fe), Chem. Mater., 2016, 28, 4129–4133 CrossRef CAS.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and beyond, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed.
- F.-X. Ma, H. Hu, H. Bin Wu, C.-Y. Xu, Z. Xu, L. Zhen and X. W. David-Lou, Formation of Uniform Fe3O4 Hollow Spheres Organized by Ultrathin Nanosheets and Their Excellent Lithium Storage Properties, Adv. Mater., 2015, 27, 4097–4101 CrossRef CAS PubMed.
- Z. Feng, X. Chen, L. Qiao, A. L. Lipson, T. T. Fister, L. Zeng, C. Kim, T. Yi, N. Sa, D. L. Proffit, A. K. Burrell, J. Cabana, B. J. Ingram, M. D. Biegalski, M. J. Bedzyk and P. Fenter, Phase-Controlled Electrochemical Activity of Epitaxial Mg-Spinel Thin Films, ACS Appl. Mater. Interfaces, 2015, 7, 28438–28443 CrossRef CAS PubMed.
- S. Liu, S. Sun and X.-Z. You, Inorganic nanostructured materials for high performance electrochemical supercapacitors, Nanoscale, 2014, 6, 2037 RSC.
- D. S. Mathew and R.-S. Juang, An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions, Chem. Eng. J., 2007, 129, 51–65 CrossRef CAS.
- C. Granados-Miralles, M. Saura-Múzquiz, H. L. Andersen, A. Quesada, J. V. Ahlburg, A.-C. Dippel, E. Canévet and M. Christensen, Approaching Ferrite-Based Exchange-Coupled Nanocomposites as Permanent Magnets, ACS Appl. Nano Mater., 2018, 1, 3693–3704 CrossRef CAS.
- A. Quesada, F. Rubio-Marcos, J. F. Marco, F. J. Mompean, M. García-Hernández and J. F. Fernández, On the origin of remanence enhancement in exchange-uncoupled CoFe2O4-based composites, Appl. Phys. Lett., 2014, 105, 202405 CrossRef.
- J. Ou-Yang, Y. Zhang, X. Luo, B. Yan, B. Zhu, X. Yang and S. Chen, Composition dependence of the magnetic properties of CoFe2O4/CoFe2 composite nano-ceramics, Ceram. Int., 2015, 41, 3896–3900 CrossRef CAS.
- A. Quesada, G. Delgado, L. Pascual, A. M. Aragón, P. Marín, C. Granados-Miralles, M. Foerster, L. Aballe, J. E. Prieto, J. de la Figuera, J. F. Fernández and P. Prieto, Exchange-spring behavior below the exchange length in hard-soft bilayers in multidomain configurations, Phys. Rev. B, 2018, 98, 214435 CrossRef CAS.
- J. V. Ahlburg, E. Canévet and M. Christensen, Air-heated solid–gas reaction setup for in situ neutron powder diffraction, J. Appl. Crystallogr., 2019, 52, 761–768 CrossRef CAS.
- Z. Guan, J. Jiang, N. Chen, Y. Gong and L. Zhen, Carbon-coated CoFe2-CoFe2O4 composite particles with high and dual-band electromagnetic wave absorbing properties, Nanotechnology, 2018, 29, 305604 CrossRef.
- M. Lu, M. Liu, L. Wang, S. Xu, J. Zhao and H. Li, Structural and magnetic properties of CoFe2O4/CoFe2/SiO2 nanocomposites with exchange coupling behavior, J. Alloys Compd., 2017, 690, 27–30 CrossRef CAS.
- R. Safi, A. Ghasemi and R. Shoja-Razavi, The role of shell thickness on the exchange spring mechanism of cobalt ferrite/iron cobalt magnetic nanocomposites, Ceram. Int., 2017, 43, 617–624 CrossRef CAS.
- J. M. Soares, F. a. O. Cabral, J. H. de Araújo and F. L. A. Machado, Exchange-spring behavior in nanopowders of CoFe2O4-CoFe2, Appl. Phys. Lett., 2011, 98, 072502 CrossRef.
- A. Quesada, C. Granados-Miralles, A. López-Ortega, S. Erokhin, E. Lottini, J. Pedrosa, A. Bollero, A. M. Aragón, F. Rubio-Marcos, M. Stingaciu, G. Bertoni, C. de Julián Fernández, C. Sangregorio, J. F. Fernández, D. Berkov and M. Christensen, Energy Product Enhancement in Imperfectly Exchange-Coupled Nanocomposite Magnets, Adv. Electron. Mater., 2016, 2, 1500365 CrossRef.
- M. Stingaciu, H. L. Andersen, C. Granados-Miralles, A. Mamakhel and M. Christensen, Magnetism in CoFe2O4 nanoparticles produced at sub- and near-supercritical conditions of water, CrystEngComm, 2017, 19, 3986–3996 RSC.
- J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Phys. B, 1993, 192, 55–69 CrossRef.
- P. Thompson, D. E. Cox and J. B. Hastings, Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3, J. Appl. Crystallogr., 1987, 20, 79–83 CrossRef CAS.
- V. Lopez-Dominguez, A. Quesada, J. C. Guzmán-Mínguez, L. Moreno, M. Lere, J. Spottorno, F. Giacomone, J. F. Fernández, A. Hernando and M. A. García, A simple vibrating sample magnetometer for macroscopic samples, Rev. Sci. Instrum., 2018, 89, 034707 CrossRef CAS.
- I. Ohnuma, H. Enoki, O. Ikeda, R. Kainuma, H. Ohtani, B. Sundman and K. Ishida, Phase equilibria in the Fe–Co binary system, Acta Mater., 2002, 50, 379–393 CrossRef CAS.
- H. Zhang, D. Zeng and Z. Liu, The law of approach to saturation in ferromagnets originating from the magnetocrystalline anisotropy, J. Magn. Magn. Mater., 2010, 322, 2375–2380 CrossRef CAS.
- C. N. Chinnasamy, B. Jeyadevan, K. Shinoda, K. Tohji, D. J. Djayaprawira, M. Takahashi, R. J. Joseyphus and A. Narayanasamy, Unusually high coercivity and critical single-domain size of nearly monodispersed CoFe2O4 nanoparticles, Appl. Phys. Lett., 2003, 83, 2862–2864 CrossRef CAS.
- M. Kahnes, R. Müller and J. Töpfer, Phase formation and magnetic properties of CoFe2O4/CoFe2 nanocomposites, Mater. Chem. Phys., 2019, 227, 83–89 CrossRef CAS.
- Y. Zhang, R. Xiong, Z. Yang, W. Yu, B. Zhu, S. Chen and X. Yang, Enhancement of Interparticle Exchange Coupling in CoFe2O4/CoFe2 Composite Nanoceramics Via Spark Plasma Sintering Technology, J. Am. Ceram. Soc., 2013, 96, 3798–3804 CrossRef CAS.
- J. M. Soares, O. L. A. Conceição, F. L. A. Machado, A. Prakash, S. Radha and A. K. Nigam, Magnetic couplings in CoFe2O4/FeCo–FeO core–shell nanoparticles, J. Magn. Magn. Mater., 2014, 374, 1–5 Search PubMed.
- F. L. A. Machado, J. M. Soares, O. L. A. Conceição, E. S. Choi and L. Balicas, Magnetic properties of the nanocomposite CoFe2O4/FeCo-FeO at a high H/T regime, J. Magn. Magn. Mater., 2017, 424, 323–326 CrossRef CAS.
- Y. Zhang, Z. Yang, B. Zhu, S. Chen, X. Yang, R. Xiong and Y. Liu, Exchange-spring effect in CoFe2O4/CoFe2 composite nano-particles, J. Alloys Compd., 2013, 567, 73–76 CrossRef CAS.
- X. Sun, Y. Q. Ma, Y. F. Xu, S. T. Xu, B. Q. Geng, Z. X. Dai and G. H. Zheng, Improved magnetic performance at low and high temperatures in non-exchange-coupling CoFe2O4/CoFe2 nanocomposites, J. Alloys Compd., 2015, 645, 51–56 CrossRef CAS.
- J. Jin, X. Sun, M. Wang, Z. L. Ding and Y. Q. Ma, The magnetization reversal in CoFe2O4/CoFe2 granular systems, J. Nanopart. Res., 2016, 18, 383 CrossRef.
- G. Du and S. Wang, Synthesis of magnetically exchange coupled CoFe2O4/CoFe2 core/shell composite particles through spray pyrolysis, J. Alloys Compd., 2017, 708, 600–604 CrossRef CAS.
- Y. Zhang, B. Yan, J. Ou-Yang, B. Zhu, S. Chen, X. Yang, Y. Liu and R. Xiong, Magnetic properties of core/shell-structured CoFe2/CoFe2O4 composite nano-powders synthesized via oxidation reaction, Ceram. Int., 2015, 41, 11836–11843 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qm00713j |
| ‡ Current address: School of Chemistry, University of Sydney, F11, NSW 2006 Sydney, Australia. |
| § Current address: School of Chemistry, UNSW Australia, NSW 2052 Sydney, Australia. |
| This journal is © the Partner Organisations 2020 |