The application of hollow micro-/nanostructured cathodes for sodium-ion batteries
Xiao-Hao
Liu
 a,
Wei-Hong
Lai
b and
Shu-Lei
Chou
a,
Wei-Hong
Lai
b and
Shu-Lei
Chou
 *ab
*ab
aSchool of Environmental and Chemical Engineering, Shanghai University, 99 Shangda Road, Shanghai, 200444, P. R. China
bInstitute for Superconducting & Electronic Materials, University of Wollongong, Innovation Campus, Wollongong, NSW 2500, Australia. E-mail: shulei@uow.edu.au
First published on 30th December 2019
Abstract
High-performance sodium-ion batteries (SIBs) rely on efficient cathodes, constructed based on advances in excellent rate capability, high capacity, and small volume change. Cathode materials with micro-/nano-hollow architectures can shorten the diffusion length, offer volume buffering, and increase the conducting capability of electrons/ions, so they have been extensively developed as a new strategy for the exploration of future cathodes. This review offers a snapshot of the recent progress on the synthetic strategies used to prepare hollow cathodes, their improved battery performance, and the mechanism of sodium storage underlying the use of hollow micro-/nanostructured cathode materials. Finally, we provide an overview of likely future directions in this research, which includes seeking the right balance between volumetric capacity and energy density.
1. Introduction
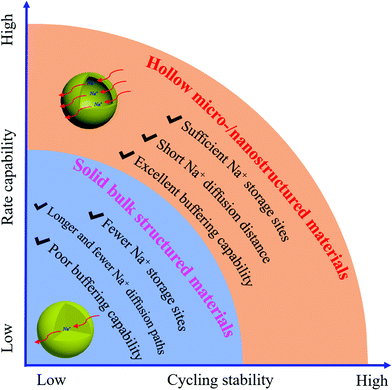
Recently, sodium-ion batteries (SIBs) have emerged as the most promising candidates for next-generation commercial energy storage systems due to the abundance of sodium resources, and the low cost and environmental-friendliness of sodium as a raw material.1–5 Many efforts have been made to develop high-performance sodium ion storage systems with high-safety, high energy density, excellent rate capability, and long cycling life. The cathode materials play a crucial role in SIBs, but the ones currently available are still challenging for real use, owing to their poor electronic/ionic conductivity and drastic volume changes during sodiation/desodiation, as well as their slow ion diffusion caused by larger particles.6–8 In order to address the above problems, several strategies have been investigated, including nanosizing the cathode materials, carbon coating, and metal ion doping.9–12 Nanoengineering cathode materials is of high significance for enhancing the cycling stability and rate capability, as the ultrathin shell and high active surface area of hollow micro-/nanostructures can accelerate the ion transport between the layers, improve the ion adsorption, and feature faster surface redox reactions.13–15 Also, the construction of hollow micro-/nanostructures offers great “breathability”, large void space, and better mono-dispersity, which are very crucial to address the issues arising from the volume changes.16 For example, Huang et al. reported hollow 0.3Li2MnO3·0.7LiNi0.5Mn0.5O2 microspheres that displayed far better rate capability and cyclability than their bulk solid particle counterpart due to the merits of hollow micro-/nanostructures.17 As shown in Fig. 1, these hollow micro-/nanostructures with cavities and void space can dramatically enhance the rate capability and cycling stability of electrodes due to having sufficient ion storage sites, short ion diffusion lengths, large contact area of the active materials with the electrolyte, and the reversible accommodation of large volume changes. In contrast, their corresponding bulk structures demonstrated inferior electrochemical performance, owing to their having fewer ion storage sites and longer ion diffusion paths as well as poor buffering capability. More research results have vitrified this; for instance, Cao et al. compared hollow micro-/nanostructured LiNi1/3Co1/3Mn1/3O2 and its bulk structure, finding that the rate and cycling performance of the former was more excellent than the latter which benefited from the reducing path of Li ion diffusion, increasing contact area between electrodes and electrolyte and buffering of the volume changes during the Li ion intercalation/deintercalation processes.18 The rational design of hollow micro-/nanostructured cathode materials and understanding their electrochemical mechanisms, thus, are beneficial for the improvement of the electrochemical performance of SIBs. Notably, the tap density of hollow micro-/nanostructured electrode materials will cause low volumetric energy density, which would limit their practical applications.19 Therefore, finding a way to find the right balance between the excellent electrochemical performance of hollow cathodic electrodes in SIBs and their modest volumetric energy density is very important. For hollow cathode materials, designing multi-shell structures may achieve higher volumetric energy density than its single-shell counterpart due to the utilization of the inner void. In this regard, Wang's group has contributed a lot of work on the illustration of the unique advantages of multi-shell hollow structured electrode materials, including LIBs and SIBs.20–22Great attention has been devoted to designing hollow micro-/nanostructured anodes for SIBs,23–26 but in the case of cathodes, there are no special reviews summarizing their progress. Herein, we summarize recent progress in the development of hollow-structured cathodes for SIBs by presenting the design strategies for the fabrication of hollow-structured cathodes and their application in SIBs. The mechanisms underlying their high electrochemical performance are also discussed in this review. Finally, a brief outlook on the future trends and challenges in this area is presented.
2. Synthesis of hollow micro-/nanostructured materials
Generally, hard-templating, soft-templating, sacrificial-templating, and template-free methods have been widely applied in the fabrication of electrode materials with hollow micro-/nanostructures. The hard-templating method has been commonly used for preparing hollow cathodes. Typically, some prepared particles need to be used as the “hard” core templates and are coated with the targeted materials or precursors. The core will then be removed via etching or thermal decomposition, and the shell layer will be retained to form a hollow structure. Polymers, silica, and carbon colloidal spheres are commonly employed as hard templates to construct hollow structures.27 The hard-templating method as a conventional strategy to construct hollow micro-/nanostructures due to its unique advantages of universality and easy-controlling size distribution. Nevertheless, the hard-templating route often needs toxic reactants, multiple steps and time-consuming processes to remove the template. The soft-templating method has attracted the greatest attention and significant progress has been made in the past decade due to the merits of flexibility, and time-saving. This method can be explained as the direct generation of hollow structures through the self-assembly of some structure guiding agents, such as surfactants or certain organic additives. As for the soft-templating method, the corresponding templates are rather easy to remove, but the morphology of the as-prepared hollow materials is usually not uniform because of their high sensitivity to the reaction conditions. What is more, the deformability and the dynamics of soft templates leads to poor monodispersity.28 Thus, it is of great significance to develop more efficient and simpler sacrificial-templating synthesis methods to prepare hollow micro-/nanostructures. The sacrificial-templating method refers to the formation of hollow micro-/nanostructures arising from the self-sacrifice of in situ formed template without the use of a pre-existing template in the reaction process.29,30 Similar to traditional hard templates, the shape and cavity size of the obtained hollow micro-/nanostructures can be directly determined by sacrificial templates, whereas the template can also play the role of a structure-directing scaffold and precursor for the shell.31 Thus, a sacrificial-templating method is inherently advantageous because it generally does not need additional surface functionalization and ensures the shell formation via a chemical reaction. In this regard, the preparation process of hollow micro-/nanostructures by this method is therefore typically more efficient. Conventional ways to fabricate hollow micro-/nanostructures generally require various pre-synthetic templates including a hard template, soft template and sacrificial template, which results in a high cost of template materials and the complexity of multi-step synthetic procedures.32 Thus, exploring template-free methods for the preparation of hollow micro-/nanostructures is of interest and remains challenging. The template-free method means that no template is required in the preparation process, and the formation mechanism can be explained by an oriented aggregation of nanoparticles self-assembling into the hollow micro-/nanostructure, such as inside-out Ostwald ripening, the Kirkendall effect and the oriented attachment process.33,34 Recently, template-free hydrothermal/solvothermal route and spray pyrolysis have been regarded as highly efficient and facile strategies to prepare hollow micro-/nanostructured electrode materials, and are easy to scale-up.35–37Fig. 2 summarizes the construction methods and their advantages and disadvantages of hollow micro-/nanostructures for different kinds of cathode materials.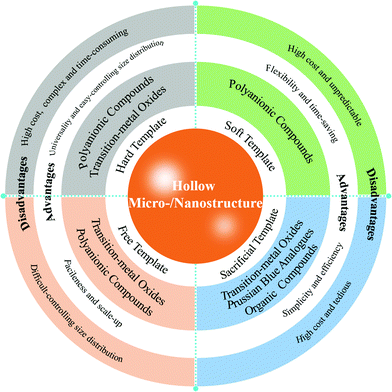 | ||
| Fig. 2 Construction methods, advantages and disadvantages of hollow micro-/nanostructures for different kinds of cathode materials. | ||
3. Sodium cathodes based on hollow micro-/nanostructured materials
Recently, many researchers have focused their attention on morphology control in energy storage materials, which is expected to significantly improve the electrochemical performance of electrode materials. Hollow micro-/nanostructures have been widely applied in cathode materials for lithium-ion batteries (LIBs), which have been regarded as effective approaches to enhance their Li storage performance.17,38–40 For example, due to the inferior electrochemical reaction kinetics of SIBs compared to LIBs, designing unique hollow structured electrodes is more essential for accelerating the migration of Na+ ions and thus achieving superior Na storage capability. Cathode materials with hollow micro-/nanostructures have attracted considerable attention for increasing the electrochemical performance of LIBs. Although they have benefited from this research, cathode materials for SIBs with hollow characteristics still need to be thoroughly explored. This work has motivated us to exploit the Na storage performance of different kinds of cathodes with hollow micro-/nanostructures. As summarized in Table 1, numerous studies about the utilization of hollow micro-/nanostructures as sodium-ion battery cathodes have been reported in recent years. In this part, we illustrate a variety of hollow micro-/nanostructured cathode materials that have been successfully used for SIBs, including transition-metal oxides, polyanionic compounds, Prussian blue analogues, and organic compounds.| Hollow cathodes | Morphology | Synthesis method | Capacity (mA h g−1) | Rate capability (mA h g−1) | Cycling performance (mA h g−1) | Ref. |
|---|---|---|---|---|---|---|
| VOOH | Microsphere | Free template | 150/0.02 A g−1 | 84/0.3 A g−1 | 126/200th/0.04 A g−1 | 45 |
| V2O5 | Nanosphere | Free template | ∼150/0.02 A g−1 | ∼112/0.64 A g−1 | 141/100th/0.02 A g−1 | 46 |
| K0.27MnO2 | Nanosphere | Hard template | 83/0.2 A g−1 | 56.6/0.6 A g−1 | 68.7/100th/0.2 A g−1 | 47 |
| Na0.7MnO2.05@PPy | Nanosphere | Sacrificial template | 165.1/0.1 A g−1 | 100.5/2 A g−1 | 142.6/100th/0.1 A g−1 | 48 |
| Na3V2(PO4)3/C | Nanosphere | Free template | 106/11.8 mA g−1 | 84/47.2 A g−1 | 93/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th/118 mA g−1 000th/118 mA g−1 |
60 |
| Na3V2(PO4)3/C | Nanosphere | Free template | ∼100/0.02 A g−1 | 75.8/0.2 A g−1 | 89.3/300th/0.02 A g−1 | 61 |
| NaFePO4 | Nanosphere | Hard template | 152.1/15.5 mA g−1 | 67.4/1.55 A g−1 | 144.3/300th/15.5 mA g−1 | 62 |
| Na2FePO4F | Nanosphere | Free template | 89/12.4 mA g−1 | 75/124 mA g−1 | 60/750th/124 mA g−1 | 63 |
| Na3V1.95Mn0.05(PO4)2F3@C | Microsphere | Free template | 122.9/25.6 mA g−1 | 60.7/1.28 A g−1 | 109/500th/25.6 mA g−1 | 64 |
| Na2FePO4F/C | Microsphere | Soft template | 120.1/12.4 mA g−1 | 65.2/0.62 A g−1 | 82.9/200th/124 mA g−1 | 65 |
| Na1.38Ni0.07Mn0.93[Fe(CN)6]0.82·□0.18·1.4H2O | Nanocube | Sacrificial template | 123/0.05 A g−1 | 52/3.2 A g−1 | 102/600th/0.05 A g−1 | 71 |
| Na0.99Mn0.37Fe0.63[Fe(CN)6]0.96·□0.04·1.36H2O | Rod-like | Sacrificial template | 117.3/0.1 A g−1 | 92.4/2 A g−1 | 109.2/300th/0.5 A g−1 | 72 |
| Na1.58Fe[Fe(CN)6]0.92 | Nanosphere | Sacrificial template | 142/17 mA g−1 | 76/1.7 A g−1 | ∼102/800th/0.34 A g−1 | 73 |
| Polyaniline | Nanofiber | Sacrificial template | 153/45 mA g−1 | 70/1.2 A g−1 | ∼66/1000th/0.8 A g−1 | 77 |
| Polypyrrole | Nanosphere | Sacrificial template | 97/0.02 A g−1 | 87/0.32 A g−1 | 77/100th/0.04 A g−1 | 78 |
3.1 Transition-metal oxides
Transition-metal oxides are the most studied cathodes owing to their high theoretical capacity and working voltage. The structure of transition metal oxides is unstable, however, which results in poor cycling stability.41–44 Hollow micro-/nanostructured electrodes with large interior free volume can alleviate the structural strains associated with repeated Na+ ion insertion/extraction processes, leading to better structural and cycling stability. Thus, constructing hollow micro-/nanostructures for achieving transition-metal oxides with high performance is a good choice. In the case of the transition-metal oxides, hollow micro-/nanostructures can be achieved by hard-templating, sacrificial-templating, and template-free methods.Zheng et al. prepared VOOH hollow microspheres with low crystallinity by a template-free hydrothermal route, and found that VOOH used as a cathode for SIBs exhibited outstanding rate performance and long cycle life.45 The low crystallinity and hollow microspherical structure of VOOH contributed to its outstanding performance, which could avoid unfavorable multiple phase transitions and alleviate the strains that appear during the insertion/extraction of Na+ ions. Furthermore, Wang's group constructed V2O5 hollow nanospheres via a template-free polyol-induced solvothermal process.46 The V2O5 hollow nanospheres exhibited more outstanding electrochemical performance compared to solid V2O5 nanocrystals. Recently, Huang et al. used mono-dispersed nanospherical polystyrene (PS) as a hard template to prepare hollow K0.27MnO2 nanospheres as a cathode material for aqueous SIBs (Fig. 3a).47 Flowerlike core–shell structured PS@K-δ-MnO2 spheres as intermediate products were obtained after a hydrothermal process. The PS template can be removed during the heating process to obtain the hollow K0.27MnO2 nanospheres. The K0.27MnO2 nanospheres are hollow and hierarchical structures composed of stacked multilayer nanosheets (Fig. 3b and c). When the hollow K0.27MnO2 nanospheres were applied as a cathode, NaTi2(PO4)3 as an anode, and 1 mol L−1 Na2SO4 aqueous solution as an electrolyte, their electrochemical performance in aqueous SIBs was investigated. Hollow K0.27MnO2 could achieve a discharge capacity of 56.6 mA h g−1 with the current density of 600 mA g−1, which represents outstanding high rate capability. Compared with the cycling performance of solid K0.27MnO2, the hollow K0.27MnO2 demonstrated a reversible discharge capacity of 68.7 mA h g−1 after 100 cycles (Fig. 3d). In contrast, only a low discharge capacity of 50 mA h g−1 was observed at 200 mA g−1 for solid K0.27MnO2 nanoparticles (Fig. 3e), indicating that constructing hollow structured K0.27MnO2 is crucial for achieving great electrochemical performance. The improvement of their Na storage property can be ascribed to these three aspects: (i) a short ion diffusion distance exists due to the thin shell, and the inner void accelerates the migration of Na+ ions; (ii) the large contact area between electrode and electrolyte ensures sufficient electrochemically active sites; and (iii) the stacked multilayer nanosheets increase the stability of the layered structure and lead to enhanced electronic conductivity, which is favorable for the charge transport and cycling stability of the electrode. Furthermore, Tu et al. showed a more direct comparison of the performances of solid and hollow electrodes.48 They fabricated hollow Na0.7MnO2.05 nanospheres (NMOHS) by using hollow MnO2 as the sacrificial template (Fig. 3f and g). In order to further enhance the electronic conductivity, the NMOHS were coated by polypyrrole (PPy) (Fig. 3h). As shown in Fig. 3i, the rate performances of hollow NMOHS@PPy and NMOHS exhibited more excellent rate capability than that of solid K0.7MnO2.05 microspheres (NMOSS). Also, the hollow NMOHS cathode demonstrated higher capacity retention of 72.7%, compared to 35.6% for the solid NMOSS cathode after 100 cycles (Fig. 3j). Furthermore, the NMOHS cathode delivered a discharge capacity of 72.5 mA h g−1 at 2 A g−1, whereas for the NMOSS cathode, the discharge capacity only remained at 29.6 mA h g−1 at the same current density. After the surface coating, the NMOHS@PPy cathode could reach 100.5 mA h g−1 at the same rate, much higher than for bare NMOHS. Thus, the construction of hollow micro-/nanostructures and PPy coating are crucial for the superior cycling stability and rate capability of the NMOHS@PPy cathode due to the enhanced conductivity and ideal contact area with the electrolyte.
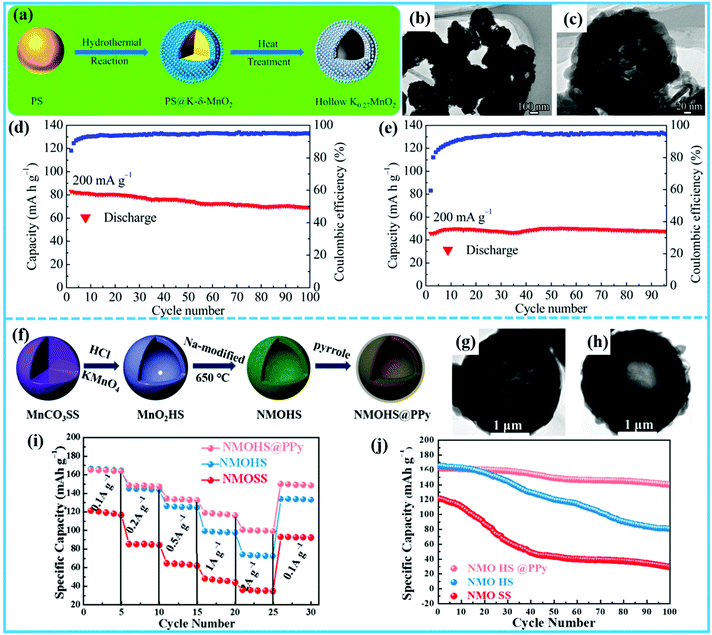 | ||
| Fig. 3 (a) Schematic illustration of the fabrication of the hollow K0.27MnO2 nanospheres. (b and c) Transmission electron microscope (TEM) images of hollow K0.27MnO2, and cycling performances of hollow K0.27MnO2 (d) and K0.27MnO2 without a PS template (e) at a current density of 200 mA g−1.47 (Reprinted with permission from the American Chemical Society. Copyright 2016.) (f) Schematic illustration of the fabrication of NMOHS and NMOHS@PPy. TEM images of NMOHS (g) and NMOHS@PPy (h), and comparison with NMOSS of (i) their rate capabilities at 0.1, 0.2, 0.5, 1, and 2 A g−1, and (j) their cycling performances at 0.1C.48 (Reprinted with permission from the American Chemical Society. Copyright 2019.). | ||
To sum up, designing hollow micro-/nanostructures can shorten the Na+ ion diffusion distance and increase the contact area of transition-metal oxide active materials with an electrolyte, thus the rate capability and cycling stability can be enhanced. What can't be ignored is that the decrease of volumetric energy density. Considering the advantage of high reversible capacity, it is still very meaningful to construct hollow micro-/nanostructures to boost the Na storage performance. In terms of practical application, multi-shelled hollow micro-/nanostructures can be a good choice.
3.2 Polyanionic compounds
In recent years, polyanionic compounds have received increasing attention and are expected to become promising cathode materials for SIBs owing to their structural stability.9,49–51 Nevertheless, their poor electronic conductivity and slow reaction kinetics lead to unsatisfactory rate performance and fast capacity decay, which severely restricts their practical applications.52,53 With the aim of overcoming these issues, hollow micro-/nanostructures can be a perfect electrode for shortening the diffusion distance of Na+ ions to improve the rate capability and cycling stability. Hollow micro-/nanostructured polyanionic compounds can be realized by hard-templating, soft-templating, and template-free methods.Recently, sodium superionic conductor (NASICON)-type Na3V2(PO4)3 (NVP), serving as representative of polyanionic compounds, has been intensively studied owing to its fast ion diffusion capability and thermal stability.54,55 Unfortunately, it suffers from poor electronic conductivity and slow reaction kinetics, which significantly limit its electrochemical performance and further practical applications.6,56–59 The electronic conductivity of NVP can be improved through carbon coating, while designing hollow micro-/nanostructures can further enhance its overall performance. For instance, Wang’ group reported three-dimensional (3D) NVP-based hollow porous nanospheres (3DHP-NVP@C) synthesized by a template-free method60 as shown in Fig. 4a and b. When employed as a sodium ion cathode, this material displayed extraordinary high-rate performance and ultralong–cycling capability. Compared with the rate capabilities of 3DHP-NVP@C, NVP@C, and NVP, the 3DHP-NVP@C electrode presented the best Na storage properties in terms of both high capacity and high rate capability (Fig. 4c). The most attractive property of the 3DHP-NVP@C was its excellent cycling stability at a high rate, achieving an ultralong cycling life of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under an ultrahigh rate of 50C with a capacity retention of over 80%. In contrast, both the NVP and NVP/C electrodes showed rapid and large capacity losses (Fig. 4d). In general, the reasons why 3DHP-NVP@C manifested better rate capability and cycling stability than the bulks were attributed to the composite's highly stable framework, porous hollow nanosphere structure, and in situ surface carbon coating. Additionally, Wang et al. applied a template-free ultrasonic spray pyrolysis process for the scalable synthesis of NVP/C porous hollow spheres (Fig. 4e and f), whereas irregular bulk particles were produced by the sol–gel method.61 As shown in Fig. 4g and h, the rate capability and cycling stability of the porous hollow NVP/C composite prepared via spray pyrolysis were higher than those of its bulk particle counterpart produced by the sol–gel method, which indicates that the feasible and controllable spray pyrolysis method is more suitable for large-scale production of high performance hollow structured cathode materials.
000 cycles under an ultrahigh rate of 50C with a capacity retention of over 80%. In contrast, both the NVP and NVP/C electrodes showed rapid and large capacity losses (Fig. 4d). In general, the reasons why 3DHP-NVP@C manifested better rate capability and cycling stability than the bulks were attributed to the composite's highly stable framework, porous hollow nanosphere structure, and in situ surface carbon coating. Additionally, Wang et al. applied a template-free ultrasonic spray pyrolysis process for the scalable synthesis of NVP/C porous hollow spheres (Fig. 4e and f), whereas irregular bulk particles were produced by the sol–gel method.61 As shown in Fig. 4g and h, the rate capability and cycling stability of the porous hollow NVP/C composite prepared via spray pyrolysis were higher than those of its bulk particle counterpart produced by the sol–gel method, which indicates that the feasible and controllable spray pyrolysis method is more suitable for large-scale production of high performance hollow structured cathode materials.
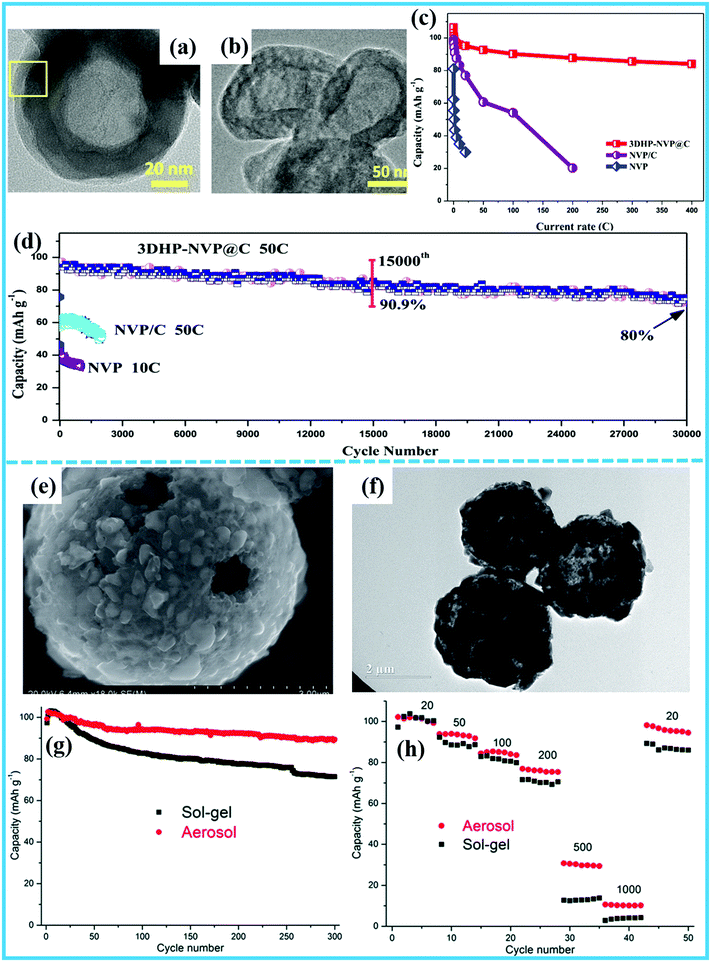 | ||
| Fig. 4 (a) TEM image. (b) High-resolution TEM (HRTEM) image (of the rectangle in (a)) of an individual hollow 3DHP-NVP@C nanosphere. (c and d) Comparison of rate performance and high-rate stability of 3DHP-NVP@C with NVP and NVP/C.60 (Reprinted with permission from Elsevier. Copyright 2017.) (e) SEM image and (f) TEM image of the aerosol synthesized Na3V2(PO4)3/C composite. (g) Rate and (h) cycling performances of the NVP/C composite prepared by spray pyrolysis (SP) and sol–gel processes.61 (Reprinted with permission from The Royal Society of Chemistry. Copyright 2015.) | ||
Similarly, NaFePO4 has the same issues of low electronic conductivity. As an analogue of LiFePO4, designing hollow micro-/nanostructured NaFePO4 cathode materials can be a very effective way to achieve high sodium storage performance. Recently, Tong et al. prepared hollow amorphous NaFePO4 nanospheres on a large scale by a simple in situ hard template process, and they proposed the possible formation mechanism of the hollow nanospheres.62 Firstly, the Fe nanospheres as a hard template can be formed by heating iron(II) stearate, and then the Fe nanospheres were further reacted with NaH2PO4 to obtain the hollow structured NaFePO4 (Fig. 5a). The as-prepared NaFePO4 composite exhibited exceptional performance with an excellent rate capability at 10C and ultrastable capacity retention over 300 cycles (Fig. 5b and c). The outstanding electrochemical properties of the hollow amorphous NaFePO4 nanospheres were attributed to its unique hollow structure, which significantly improved the ionic and electronic transport and the de-intercalation/intercalation kinetics of Na+ ions. Numerous studies have proved that the hollow structured cathodes are crucial for good electrochemical performance. Wang et al. reported carbon-coated porous hollow Na2FePO4F synthesized by a one-step template-free ultrasonic spray pyrolysis process.63 Benefiting from its unique porous hollow structure, the electrochemical reaction can take place on both the outside and inside surfaces and in the pores. After 750 cycles at 1C, Na2FePO4F/C cathodes can still provide a high capacity of 60 mA h g−1, showing excellent Na storage performance. Additionally, uniform hierarchical Na3V1.95Mn0.05(PO4)2F3@C hollow microspheres were prepared by Xu et al.64 Due to the synergistic effects of the hierarchical hollow microspherical structure, the Mn2+ doping, and the moderate carbon coating, the cathode materials displayed enhanced electrochemical performance. In order to further improve the electrochemical performance, multi-shelled hollow structures were also prepared. Sun's group synthesized hollow structured Na2FePO4F/C spheres via a solvothermal method using sodium dodecyl sulfonate (SDS) as the soft template.65 Interestingly, when the reaction time was prolonged, the structure of the microspheres gradually changed from urchin-like hollow structures to acanthosphere-like hollow structures and finally to double-shelled hollow structures (Fig. 5d–f). As shown in Fig. 5g, when the three samples experienced a current rate in the range of 5C to 0.1C, the capacity recovery of the double-shelled hollow Na2FePO4F/C was 97.5%, higher than for the acanthosphere-like hollow structure (95.3%) and the urchin-like hollow structure (89.7%), exhibiting much better rate capability. Additionally, the double-shelled hollow Na2FePO4F/C sample showed more stable cycling performance (92.5% capacity retention after 200 cycles), much better than for the acanthosphere-like hollow structure (86.5%) and urchin-like hollow structure (69.9%) (Fig. 5h). These results demonstrated that double-shelled hollow structures with thin walls can provide more active sites and contact area between the electrode materials and the electrolyte, and thus more outstanding electrochemical properties.
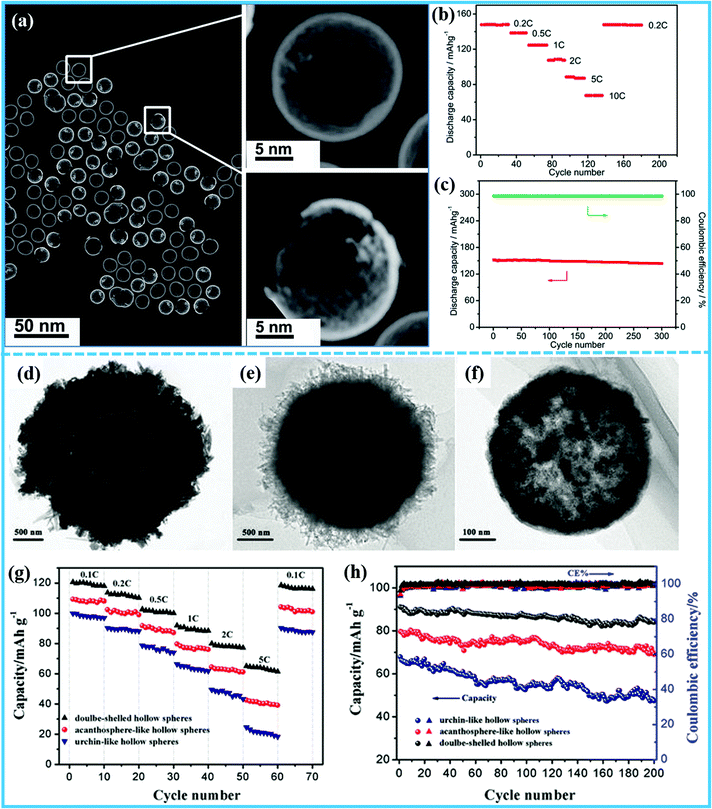 | ||
| Fig. 5 (a) Low magnification scanning TEM (STEM) image and enlarged STEM images of single nanospheres framed in (a) of the as-prepared hollow amorphous NaFePO4 nanospheres. (b and c) Rate and cycling performances of the hollow amorphous NaFePO4 nanospheres.62 (Reprinted with permission from The Royal Society of Chemistry. Copyright 2015.) (d) TEM images of the as-synthesized urchin-like hollow Na2FePO4F/C sample. (e) The acanthosphere-like hollow Na2FePO4F/C sample, and (f) the double-shelled hollow Na2FePO4F/C sample. (g and h) Rate and cycling performances of the urchin-like hollow Na2FePO4F/C, acanthosphere-like hollow Na2FePO4F/C, and double-shelled hollow Na2FePO4F/C samples.65 (Reprinted with permission from Springer, Copyright 2018.) | ||
Overall, the problems of slow reaction kinetics and fast capacity decay of polyanionic cathodes can be solved effectively by virtue of hollow micro-/nanostructures because of the shortened ion diffusion length, more contact area with electrolyte and the accommodation of volume changes. However, the challenge is the specific capacity of this kind of cathode is usually not high, and the hollow micro-/nanostructures will further lower the volumetric capacity. Designing a multi-shelled structure may relieve this awkward situation, but the hollow polyanionic cathode materials still have a long way to go for practical applications.
3.3 Prussian blue analogues
Prussian blue analogues (PBAs) have diverted the attention of many researchers from other types of materials (metal oxides, metal phosphides, metal sulfides, etc.), due to their facile synthesis method, low-cost raw precursors, and rich redox couples, which contribute to their excellent electrochemical performance.66–68 Also, PBAs are regarded as the most promising cathode materials for SIBs, which have been studied in depth by numerous researchers. They still suffer, however, from kinetic problems associated with the solid-state diffusion of Na+ ions during charge and discharge processes, which leads to low specific capacity and poor rate performance, critically obstructing their wide-scale applications.69,70 Thus, designing hollow micro-/nanostructured PBAs may be very meaningful for enhancing Na storage performance. The sacrificial-templating method can help with the formation of this unique structure.Chen et al. applied surface modification and used a hollow architecture to obtain hollow-core–shell heterostructured PBAs with Mn2+ ion and Ni2+ ion co-doping (denoted as HCS-PBMN).71 With the assistance of a high-concentration polymer as the hard template, a novel structured HCS-PBMN was formed (Fig. 6a). Through a slow nucleation process, the HCS-PBMN could be prepared with a low defect content and a high sodium content. In addition, the special hollow and core–shell structure of high-quality HCS-PBMN contributed significantly to the enhanced Na storage performance (Fig. 6b–d). As regards its electrochemical performance, HCS-PBMN exhibited a remarkable advance compared with other similar materials. The HCS-PBMN showed a high capacity of 123 mA h g−1 at 50 mA g−1, a good rate performance of 52 mA h g−1 at 3200 mA g−1, and a long cycle life with 82.3% capacity retention after 600 cycles (Fig. 6e and f). This superb electrochemical performance can be attributed not only to the hollow structure, but also to the stable PBN coating layer, in which a large buffer space and high specific surface area were conducive to relieving the volume expansion and improving the ion transfer rate. In addition, the stable PBN coating layer reduced the interface impedance and increased the ion diffusion rate in the bulk. Furthermore, Ma et al. synthesized hierarchical hollow rod-like Mn-doped Prussian blue (R-PB) using MnO2 nanosheets as a self-sacrificial template.72 When applying as a cathode for SIBs, it exhibited appealing electrochemical performance, achieving a high discharge capacity of 117.3 mA h g−1 and a capacity retention of 98.5% after 200 cycles at 1C. The enhanced discharge capacity and cycling stability of R-PB might be ascribed to the positive effects of the doped-Mn ions and the hierarchical hollow structure of R-PB.
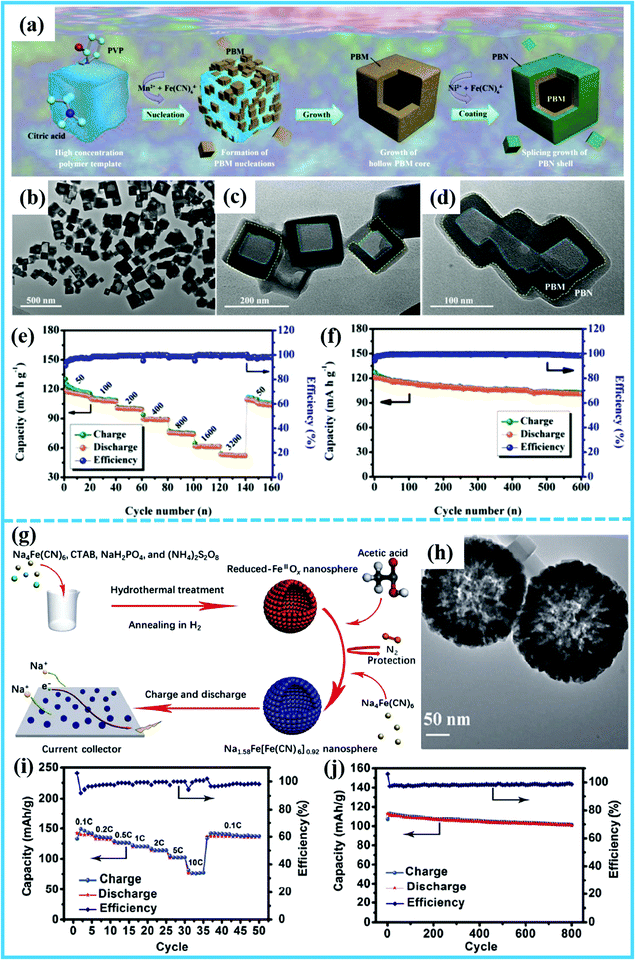 | ||
| Fig. 6 (a) Schematic illustration of the formation process of the regular heterogeneous HCS-PBMN structure. (b–d) TEM images at different magnifications of HCS-PBMN samples. (e) Rate performance of HCS-PBMN electrodes at various rates (current density: mA g−1). (f) Long-term cycling performance of HCS-PBMN electrodes measured at a current density of 50 mA g−1.71 (Reprinted with permission from Wiley-VCH Verlag Gmbh. Copyright 2018.) (g) Schematic illustration of the synthesis and the discharge mechanism of Na1.58Fe[Fe(CN)6]0.92 hollow nanosphere electrodes. (h) TEM image of Na1.58Fe[Fe(CN)6]0.92 hollow nanospheres. (i) Rate and (j) cycling performances of the Na1.58Fe[Fe(CN)6]0.92 nanosphere electrode.73 (Reprinted with permission from Springer. Copyright 2018.). | ||
Wang et al. reported hierarchical Na-rich Na1.58Fe[Fe(CN)6]0.92 hollow nanospheres with enhanced electrochemical performance.73 A schematic illustration of their synthesis is shown in Fig. 6g. The Na1.58Fe[Fe(CN)6]0.92 hollow nanospheres were prepared via a facile hydrothermal process utilizing reduced FeIIOx as a self-sacrificial template with the assistance of ascorbic acid as a reducing agent. Na1.58Fe[Fe(CN)6]0.92 hollow nanospheres with diameters of about 200 nm were obtained, as shown in the TEM image in Fig. 6h. Compared with the traditional Na-rich Na1.58Fe[Fe(CN)6]0.92 nanocube morphology, the hollow nanospheres possessed more unique features. For example, the hierarchical architecture had a larger surface area, thus introducing more active sites for the transport of electrons and Na+ ions. Furthermore, the hollow structure can buffer the volume changes during insertion/extraction of Na+ ions, thus ensuring structural stability. Thanks to these unique characteristics, the as-prepared Na1.58Fe[Fe(CN)6]0.92 nanosphere electrodes delivered an initial charge capacity of 133 mA h g−1 and discharge capacity of 142 mA h g−1, corresponding to an extraction of ∼1.4 Na and insertion of ∼1.5 Na per formula unit, respectively. A capacity of 76 mA h g−1 could be obtained even at a current density of 10C, indicating superior rate capability (Fig. 6i). Moreover, the cycling performance was also enhanced, with 90% capacity retention even after 800 cycles at 2C (Fig. 6j).
Currently, the main obstacles of PBAs are their poor electrical conductivity and slow Na+ ion diffusion. From the above reports, hollow micro-/nanostructures display superior advantages through improving the reaction kinetics and buffering the volume changes, thus, the rate and cycling performance can be enhanced. Furthermore, if more attention is paid to the increase of tap density, high-performance PBAs with hollow micro-/nanostructures will exhibit great commercialization prospects.
3.4 Organic compounds
Organic compounds have been developed as promising substitutes for inorganic cathodes in rechargeable SIBs due to their high theoretical capacities, rapid reaction kinetics, mechanical flexibility, environmental friendliness, and structural diversity.74–76 Because of the intrinsic electrically insulating properties of conjugated carbonyl polymers, it is a great challenge to simultaneously achieve high capacity, high rate capability, and long cycle life.12,75 Therefore, constructing hollow micro-/nanostructured composites is expected to be an effective way to obtain higher performance. The sacrificial-templating method has been used to construct hollow micro-/nanostructured organic compounds.For example, Cao et al. reported a facile and controllable sacrificial-template strategy combined with the electrospinning method to fabricate polyaniline hollow nanofibers (PANI-HNFs) with different diameters and wall sizes (Fig. 7a).77 Here, the poly (methyl methacrylate) nanofibers (PMMA) acted as a sacrifice template (Fig. 7b). The PANI was coated on the surfaces of PMMA nanofibers through a polymerization reaction to form coaxial PMMA@PANI nanofibers (Fig. 7c), and after dissolving the PMMA in toluene, the PANI-HNFs were left (Fig. 7d and e). The PANI-HNFs as a cathode material for SIBs exhibited the high charge and discharge capacity of 230 and 180 mA h g−1 in the initial cycle, respectively (Fig. 7f). What is more, the PANI-HNFs displayed the high rate capability of 70 mA h g−1 at 8C (Fig. 7g) and high cycling stability with 73.3% capacity retention after 1000 cycles (Fig. 7h). In order to better illustrate the advantages of hollow PANI-HNFs, PANI nanoparticles were also synthesized by using a similar chemical oxidative polymerization method but without the addition of PMMA nanofibers. The product showed the granular morphology of agglomerated nanoparticles. They found that the PANI-HNFs exhibited superior rate capacity compared with the PANI nanoparticles. Overall, the PANI-HNF cathode displayed excellent cycling stability and good rate capability, which was mainly due to its unique hollow fiber structure. On the one hand, the unique hollow fiber structure increases the contact area between the cathode material and the electrolyte, and forms good electronic and ionic conductive networks. On the other hand, the numerous voids alleviate volume changes during charging and discharging. Additionally, polypyrrole (PPy) hollow nanospheres with high sodium storage capacity were synthesized using poly(methyl methacrylate) (PMMA) nanospheres as sacrificial templates by Wang's group (Fig. 7i).78 The HRTEM image of PPy hollow nanospheres confirmed that the PMMA nanosphere templates can be completely removed through simply washing with acetone and the vacant inner space can be seen clearly from the inset image (Fig. 7j). When the as-prepared PPy hollow nanospheres were used as cathodes for SIBs, they showed a surprisingly high rate capability, with reversible capacities of 100 mA h g−1 and 69 mA h g−1 at 20 mA g−1 and 320 mA g−1, respectively (Fig. 7k). Moreover, exceptional cycling stability was achieved with 78.5% capacity retention after 1000 cycles under a high current density of 400 mA g−1 (Fig. 7l). In general, the solid electrodes are likely to show higher volumetric density than the hollow electrodes, whereas the cycling and rate performance are usually inferior due to the insufficient contact area with the electrolyte and the longer ion diffusion pathways.
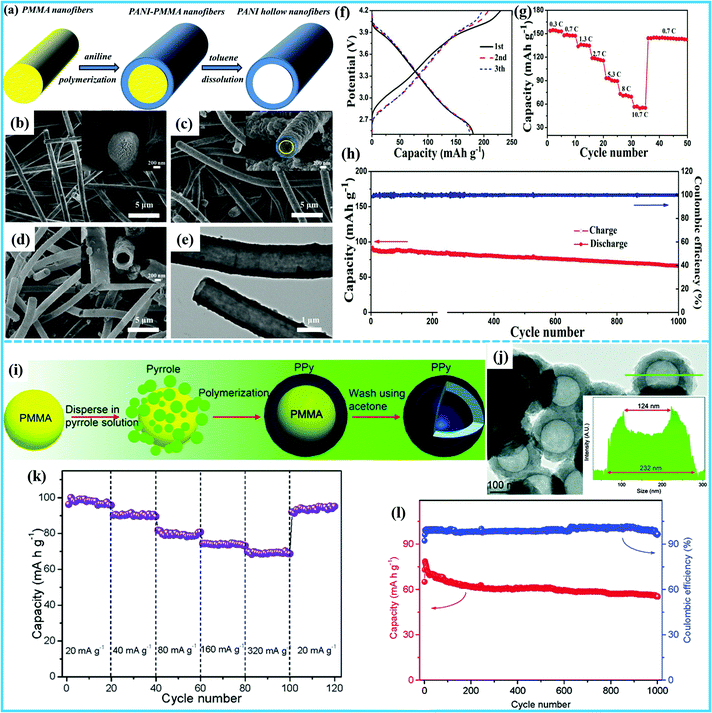 | ||
| Fig. 7 (a) Schematic illustration of the fabrication of the PANI-HNFs. Field emission SEM (FESEM) images of (b) bare PMMA nanofibers. (c) PMMA@PANI nanofibers, and (d) PANI-HNFs. (e) TEM image of PANI-HNFs. (f) Charge and discharge curves of the PANI-HNFs at 0.13C. (g) Rate performance of the PANI-HNFs. (h) Cycling performance of the PANI-HNF electrodes at 5.3C.77 (Reprinted with permission from Elsevier. Copyright 2019.) (i) Schematic illustration of the synthesis of PPy hollow nanospheres. (j) HRTEM image of PPy hollow nanospheres, electron signal counts from the cross-section of the PPy hollow nanosphere shown in the inset. (k) Rate performance of the PPy hollow nanosphere cathode at various current densities. (l) Long-term cycling performance and corresponding coulombic efficiency of PPy hollow nanospheres at a current density of 400 mA g−1.78 (Reprinted with permission from The Royal Society of Chemistry. Copyright 2015.) | ||
All in all, organic compounds applied in SIBs are promising due to their low-cost and environmentally benign organic sources. By constructing hollow micro-/nanostructures, excellent sodium storage capability can be achieved. The low volumetric capacity for polymer cathodes, however, inspires the designing of more complex hollow structures, such as multi-shelled hollow fiber structures. Therefore, multi-shelled hollow polymer electrodes could be developed for high-performance and low-cost SIBs.
4. Sodium storage mechanism of hollow micro-/nanostructured cathodes
The hollow micro-/nanostructures have been verified to be very effective in enhancing the electrochemical performance of SIBs by the above numerous reports. Further deep understanding of the sodium storage mechanism can help to realize the advantages of hollow micro-/nanostructures comprehensively.As the cathode materials of SIBs, most of them adopt the intercalation mechanism based on the reversible insertion/extraction of sodium ions. For example, from the aforementioned illustration, hierarchical Na-rich Na1.58Fe[Fe(CN)6]0.92 hollow nanospheres exhibit excellent rate and cycling performance.73 For the purpose of understanding the superior properties by constructing hollow structures, Wang et al. conducted cyclic voltammetry (CV) tests at various scan rates to calculate the capacity contribution, and found which is dominated by the intercalation process at the peak voltage. While with the increase of scan rates, the capacitive contribution increased gradually. This can explain the outstanding rate capability, which benefited from the increased surface area of Na1.58Fe[Fe(CN)6]0.92 with a hollow nanospherical structure. Furthermore, to investigate the sodium ion insertion/extraction mechanism, they obtained in situ XRD patterns of the electrode during the charge/discharge process and found that the reversible sodium insertion and extraction contributed to its excellent reversibility and cycling performance. Additionally, except for the conventional intercalation reaction mechanism, doping/de-doping reaction mechanisms can also achieve high-performance organic compounds for SIBs. To further understand the mechanism of sodium ion storage in the as-prepared PPy hollow nanospheres, Wang et al. revealed that the PPy hollow nanospheres function through reversible doping/de-doping reactions during the charge/discharge processes by virtue of the ex situ SEM, TEM, FTIR spectroscopy, and Raman spectroscopy, as well as density functional theory (DFT) calculation on the energy barriers of sodiated PPy.78 The unique hollow micro-/nanostructures can further enhance the stable cyclability of the PPy hollow nanospheres as cathode materials for SIBs.
5. Conclusion and outlook
In summary, we have reviewed the state-of-the-art research progress on hollow micro-/nanostructured cathodes for sodium-ion batteries. Due to their fascinating structural characteristics, such as larger surface area, thinner shell thickness, and larger internal space, hollow micro-/nanostructures demonstrate promising potential for use in sodium-ion batteries with abundant Na+ ion storage sites, shorter transport pathways, and improved buffering of the volume changes induced by stress/strain in successive intercalation/de-intercalation of Na+ ions. In this review article, we first covered the fabrication methods for hollow micro-/nanostructures and discussed their respective advantages. Then, the recent progress made in the application of hollow micro-/nanostructures in sodium-ion batteries was reviewed according to the different synthesis methods (hard template, soft template, sacrificial template and template-free) to elucidate the advantages of hollow micro-/nanostructures for improving the capacity, stability, and rate capability simultaneously and substantially. Lastly, the sodium storage mechanism of hollow micro-/nanostructured cathodes was introduced.Nevertheless, there are still some challenges for hollow micro-/nanostructured electrode materials within both the material synthesis and electrochemical performance, and future research is still necessary for various aspects. Firstly, what needs to be considered is that their low volumetric energy density is the main obstacle for the practical application of hollow micro-/nanostructured cathodes. Thus, seeking to balance the volumetric capacity and energy density is the key to practical hollow structured cathodes in future research. The use of multi-shelled structures may be the best approach to improving the volumetric capacity while retaining all the advantages of hollow structured materials. Secondly, there are still few reports, however, on the use of multi-shelled hollow micro-/nanostructured cathodes for sodium-ion batteries. And the relationship between the unique structural advantages and electrochemical performance of hollow micro-/nanostructures for use in SIBs need to be comprehensively and deeply understood with the help of some advanced in situ characterization and theoretical calculations, such as in situ XRD, SEM, TEM and DFT. Also, it is still difficult to fabricate high-quality multi-shelled hollow materials that satisfy the requirements of energy storage batteries. Finally, the development of large-scale, low-cost fabrication strategies for the preparation of cathodes with a desirable performance is an important challenge. Considering the inferior electronic conductivity of most cathode materials, carbon coating or hybridization with highly conductive materials have already been demonstrated as effective ways to further improve their high rate performance. The volumetric energy density and tap density of the hollow micro-/nanostructures can be relieved by the designing of multi-shelled structures to some extent; however, there are still bottlenecks toward their large-scale commercial applications and extensive exploration is needed in future research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51971124). The authors would like to thank Dr Tania Silver for critical reading of the paper.References
- T. Liu, Y. Zhang, Z. Jiang, X. Zeng, J. Ji, Z. Li, X. Gao, M. Sun, Z. Lin, M. Ling, J. Zheng and C. Liang, Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage, Energy Environ. Sci., 2019, 12, 1512–1533 RSC.
- M. Chen, Q. Liu, S. W. Wang, E. Wang, X. Guo and S. L. Chou, High-Abundance and Low-Cost Metal-Based Cathode Materials for Sodium-Ion Batteries: Problems, Progress, and Key Technologies, Adv. Energy Mater., 2019, 9, 1803609 CrossRef.
- J. Deng, W.-B. Luo, S.-L. Chou, H.-K. Liu and S.-X. Dou, Sodium-Ion Batteries: From Academic Research to Practical Commercialization, Adv. Energy Mater., 2018, 8, 1701428 CrossRef.
- X. Pu, H. Wang, D. Zhao, H. Yang, X. Ai, S. Cao, Z. Chen and Y. Cao, Recent Progress in Rechargeable Sodium-Ion Batteries: toward High-Power Applications, Small, 2019, 15, 1805427 CrossRef.
- Z. Lin, Q. Xia, W. Wang, W. Li and S. Chou, Recent research progresses in ether-and ester-based electrolytes for sodium-ion batteries, InfoMat, 2019, 1, 376–389 CrossRef CAS.
- L. Zhao, H. Zhao, Z. Du, J. Wang, X. Long and Z. Li, and K. Świerczek, Delicate lattice modulation enables superior Na storage performance of Na3V2(PO4)3 as both an anode and cathode material for sodium-ion batteries: understanding the role of calcium substitution for vanadium, J. Mater. Chem. A, 2019, 7, 9807–9814 RSC.
- J. Kim, H. Kim and K. Kang, Conversion-Based Cathode Materials for Rechargeable Sodium Batteries, Adv. Energy Mater., 2018, 8, 1702646 CrossRef.
- W.-J. Li, C. Han, W. Wang, F. Gebert, S.-L. Chou, H.-K. Liu, X. Zhang and S.-X. Dou, Commercial Prospects of Existing Cathode Materials for Sodium Ion Storage, Adv. Energy Mater., 2017, 7, 1700274 CrossRef.
- X. Liu, L. Tang, Z. Li, J. Zhang, Q. Xu, H. Liu, Y. Wang, Y. Xia, Y. Cao and X. Ai, An Al-doped high voltage cathode of Na4Co3(PO4)2P2O7 enabling highly stable 4 V full sodium-ion batteries, J. Mater. Chem. A, 2019, 7, 18940–18949 RSC.
- W. Shen, C. Wang, Q. Xu, H. Liu and Y. Wang, Nitrogen-Doping-Induced Defects of a Carbon Coating Layer Facilitate Na-Storage in Electrode Materials, Adv. Energy Mater., 2015, 5, 1400982 CrossRef.
- S. Liu, L. Wang, J. Liu, M. Zhou, Q. Nian, Y. Feng, Z. Tao and L. Shao, Na3V2(PO4)2F3-SWCNT: a high voltage cathode for non-aqueous and aqueous sodium-ion batteries, J. Mater. Chem. A, 2019, 7, 248–256 RSC.
- Y. Liang, W. H. Lai, Z. Miao and S. L. Chou, Nanocomposite Materials for the Sodium-Ion Battery: A Review, Small, 2018, 14, 1702514 CrossRef.
- L. Wu, Y. Hu, X. Zhang, J. Liu, X. Zhu and S. Zhong, Synthesis of carbon-coated Na2MnPO4F hollow spheres as a potential cathode material for Na-ion batteries, J. Power Sources, 2018, 374, 40–47 CrossRef CAS.
- K. Naoi, K. Kisu, E. Iwama, S. Nakashima, Y. Sakai, Y. Orikasa, P. Leone, N. Dupré, T. Brousse, P. Rozier, W. Naoi and P. Simon, Ultrafast charge-discharge characteristics of a nanosized core-shell structured LiFePO4 material for hybrid supercapacitor applications, Energy Environ. Sci., 2016, 9, 2143–2151 RSC.
- W.-H. Lai, Y.-X. Wang, Y. Wang, M. Wu, J.-Z. Wang, H.-K. Liu, S.-L. Chou, J. Chen and S.-X. Dou, Morphology tuning of inorganic nanomaterials grown by precipitation through control of electrolytic dissociation and supersaturation, Nat. Chem., 2019, 11, 695–701 CrossRef CAS.
- Y. Xu, W. Shen, A. Zhang, H. Liu and Z. Ma, Template-free hydrothermal synthesis of Li2FeSiO4 hollow spheres as cathode materials for lithium-ion batteries, J. Mater. Chem. A, 2014, 2, 12982–12990 RSC.
- Y. Jiang, Z. Yang, W. Luo, X. Hu and Y. Huang, Hollow 0.3Li2MnO3·0.7LiNi0.5Mn0.5O2 microspheres as a high-performance cathode material for lithium-ion batteries, Phys. Chem. Chem. Phys., 2013, 15, 2954–2960 RSC.
- J. Li, C. Cao, X. Xu, Y. Zhu and R. Yao, LiNi1/3Co1/3Mn1/3O2 hollow nano-micro hierarchical microspheres with enhanced performances as cathodes for lithium-ion batteries, J. Mater. Chem. A, 2013, 1, 11848–11852 RSC.
- F. Xie, L. Zhang, C. Ye, M. Jaroniec and S. Z. Qiao, The Application of Hollow Structured Anodes for Sodium-Ion Batteries: From Simple to Complex Systems, Adv. Mater., 2019, 31, 1800492 CrossRef.
- J. Wang, H. Tang, H. Wang, R. Yu and D. Wang, Multi-shelled hollow micro-/nanostructures: promising platforms for lithium-ion batteries, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- X. Zhao, J. Wang, R. Yu and D. Wang, Construction of Multishelled Binary Metal Oxides via Coabsorption of Positive and Negative Ions as a Superior Cathode for Sodium-Ion Batteries, J. Am. Chem. Soc., 2018, 140, 17114–17119 CrossRef CAS.
- J. Wang, H. Tang, L. Zhang, H. Ren, R. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. Liu, Y. Zhang, Y. Wen, L. Gu, G. Ma, Z. Su, Z. Tang, H. Zhao and D. Wang, Multi-shelled metal oxides prepared via an anion-adsorption mechanism for lithium-ion batteries, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
- C. Dong, J. Liang, Y. He, C. Li, X. Chen, L. Guo, F. Tian, Y. Qian and L. Xu, NiS1.03 Hollow Spheres and Cages as Superhigh Rate Capacity and Stable Anode Materials for Half/Full Sodium-Ion Batteries, ACS Nano, 2018, 12, 8277–8287 CrossRef CAS.
- F. Xie, L. Zhang, D. Su, M. Jaroniec and S.-Z. Qiao, Na2Ti3O7@N-Doped Carbon Hollow Spheres for Sodium-Ion Batteries with Excellent Rate Performance, Adv. Mater., 2017, 29, 1700989 CrossRef.
- F. Xie, L. Zhang, Q. Gu, D. Chao, M. Jaroniec and S.-Z. Qiao, Multi-shell hollow structured Sb2S3 for sodium-ion batteries with enhanced energy density, Nano Energy, 2019, 60, 591–599 CrossRef CAS.
- X. Q. Zhang, Y. C. Zhao, C. G. Wang, X. Li, J. D. Liu, G. H. Yue and Z. D. Zhou, Facile synthesis of hollow urchin-like NiCo2O4 microspheres for high-performance sodium-ion batteries, J. Mater. Sci., 2016, 51, 9296–9305 CrossRef CAS.
- C. C. Nguyen, N. N. Vu and T.-O. Do, Recent advances in the development of sunlight-driven hollow structure photocatalysts and their applications, J. Mater. Chem. A, 2015, 3, 18345–18359 RSC.
- S. Li, A. Pasc, V. Fierro and A. Celzard, Hollow carbon spheres, synthesis and applications-a review, J. Mater. Chem. A, 2016, 4, 12686–12713 RSC.
- X. Zhao, J. Zhu, W. Cai, M. Xiao, L. Liang, C. Liu and W. Xing, Pt-Pb hollow sphere networks: self-sacrifice-templating method and enhanced activity for formic acid electrooxidation, RSC Adv., 2013, 3, 1763–1767 RSC.
- X. Zhou, J. Tian, Q. Wu, J. Hu and C. Li, N/O dual-doped hollow carbon microspheres constructed by holey nanosheet shells as large-grain cathode host for high loading Li–S batteries, Energy Storage Mater., 2019, 24, 644–654 CrossRef.
- X. W. Lou, L. A. Archer and Z. Yang, Hollow Micro-/Nanostructures: Synthesis and Applications, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- X. Lai, J. E. Halpert and D. Wang, Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems, Energy Environ. Sci., 2012, 5, 5604–5618 RSC.
- Y. Yue, A. J. Binder, B. Guo, Z. Zhang, Z. A. Qiao, C. Tian and S. Dai, Mesoporous Prussian Blue Analogues: Template-Free Synthesis and Sodium-Ion Battery Applications, Angew. Chem., Int. Ed., 2014, 53, 3134–3137 CrossRef CAS.
- F. Han, C. Y. Jun Tan and Z. Gao, Template-free formation of carbon nanotube-supported cobalt sulfide@carbon hollow nanoparticles for stable and fast sodium ion storage, J. Power Sources, 2017, 339, 41–50 CrossRef CAS.
- X. Jing, Y. Zhang, H. Jiang, Y. Cheng, N. Xing and C. Meng, Facile template-free fabrication of hierarchical V2O5 hollow spheres with excellent charge storage performance for symmetric and hybrid supercapacitor devices, J. Alloys Compd., 2018, 763, 180–191 CrossRef CAS.
- H. Tong, Y. Xu, X. Cheng, X. Zhang, S. Gao, H. Zhao and L. Huo, One-pot solvothermal synthesis of hierarchical WO3 hollow microspheres with superior lithium ion battery anode performance, Electrochim. Acta, 2016, 210, 147–154 CrossRef CAS.
- F. Ghani, A. Raza, D. Kyung, H.-S. Kim, J. Lim and I. W. Nah, Optimization of synthesis conditions of high-tap density FeVO4 hollow microspheres via spray pyrolysis for lithium-ion batteries, Appl. Surf. Sci., 2019, 497, 143718 CrossRef CAS.
- F. Wang, J. Wang, H. Ren, H. Tang, R. Yu and D. Wang, Multi-shelled LiMn2O4 hollow microspheres as superior cathode materials for lithium-ion batteries, Inorg. Chem. Front., 2016, 3, 365–369 RSC.
- L. Zhou, D. Zhao and X. Lou, LiNi0.5Mn1.5O4 Hollow Structures as High-Performance Cathodes for Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2012, 51, 239–241 CrossRef CAS.
- S. Yang, M. Hu, L. Xi, R. Ma, Y. Dong and C. Y. Chung, Solvothermal Synthesis of Monodisperse LiFePO4 Micro Hollow Spheres as High Performance Cathode Material for Lithium Ion Batteries, ACS Appl. Mater. Interfaces, 2013, 5, 8961–8967 CrossRef CAS.
- C. Luo, A. Langrock, X. Fan, Y. Liang and C. Wang, P2-type transition metal oxides for high performance Na–ion battery cathodes, J. Mater. Chem. A, 2017, 5, 18214–18220 RSC.
- E. Talaie, S. Y. Kim, N. Chen and L. F. Nazar, Structural Evolution and Redox Processes Involved in the Electrochemical Cycling of P2-Na0.67[Mn0.66Fe0.20Cu0.14]O2, Chem. Mater., 2017, 29, 6684–6697 CrossRef CAS.
- L. Wang, Y.-G. Sun, L.-L. Hu, J.-Y. Piao, J. Guo, A. Manthiram, J. Ma and A.-M. Cao, Copper-substituted Na0.67Ni0.3−xCuxMn0.7O2 cathode materials for sodium-ion batteries with suppressed P2–O2 phase transition, J. Mater. Chem. A, 2017, 5, 8752–8761 RSC.
- P.-F. Wang, Y. You, Y.-X. Yin and Y.-G. Guo, Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance, Adv. Energy Mater., 2018, 8, 1701912 CrossRef.
- J. Shao, Y. Ding, X. Li, Z. Wan, C. Wu, J. Yang, Q. Qu and H. Zheng, Low crystallinity VOOH hollow microspheres as an outstanding high-rate and long-life cathode for sodium ion batteries, J. Mater. Chem. A, 2013, 1, 12404–12408 RSC.
- D. W. Su, S. X. Dou and G. X. Wang, Hierarchical orthorhombic V2O5 hollow nanospheres as high performance cathode materials for sodium-ion batteries, J. Mater. Chem. A, 2014, 2, 11185–11194 RSC.
- Y. Liu, Y. Qiao, X. Lou, X. Zhang, W. Zhang and Y. Huang, Hollow K0.27MnO2 Nanospheres as Cathode for High-Performance Aqueous Sodium Ion Batteries, ACS Appl. Mater. Interfaces, 2016, 8, 14564–14571 CrossRef CAS.
- D. Lu, Z. Yao, Y. Zhong, X. Wang, X. Xia, C. Gu, J. Wu and J. Tu, Polypyrrole-Coated Sodium Manganate Hollow Microspheres as a Superior Cathode for Sodium Ion Batteries, ACS Appl. Mater. Interfaces, 2019, 11, 15630–15637 CrossRef CAS.
- Y. Fang, J. Zhang, L. Xiao, X. Ai, Y. Cao and H. Yang, Phosphate Framework Electrode Materials for Sodium Ion Batteries, Adv. Sci., 2017, 4, 1600392 CrossRef.
- M. Chen, D. Cortie, Z. Hu, H. Jin, S. Wang, Q. Gu, W. Hua, E. Wang, W. Lai, L. Chen, S.-L. Chou, X.-L. Wang and S.-X. Dou, A Novel Graphene Oxide Wrapped Na2Fe2(SO4)3/C Cathode Composite for Long Life and High Energy Density Sodium-Ion Batteries, Adv. Energy Mater., 2018, 8, 1800944 CrossRef.
- C. Fang, Y. Huang, W. Zhang, J. Han, Z. Deng, Y. Cao and H. Yang, Routes to High Energy Cathodes of Sodium-Ion Batteries, Adv. Energy Mater., 2016, 6, 1501727 CrossRef.
- S.-P. Guo, J.-C. Li, Q.-T. Xu, Z. Ma and H.-G. Xue, Recent achievements on polyanion-type compounds for sodium-ion batteries: Syntheses, crystal chemistry and electrochemical performance, J. Power Sources, 2017, 361, 285–299 CrossRef CAS.
- S. Chen, C. Wu, L. Shen, C. Zhu, Y. Huang, K. Xi, J. Maier and Y. Yu, Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries, Adv. Mater., 2017, 29, 1700431 CrossRef.
- Q. Ni, Y. Bai, F. Wu and C. Wu, Polyanion-Type Electrode Materials for Sodium-Ion Batteries, Adv. Sci., 2017, 4, 1600275 CrossRef.
- P. Hu, X. Wang, J. Ma, Z. Zhang, J. He, X. Wang, S. Shi, G. Cui and L. Chen, NaV3(PO4)3/C nanocomposite as novel anode material for Na–ion batteries with high stability, Nano Energy, 2016, 26, 382–391 CrossRef CAS.
- Q. Zhu, X. Chang, N. Sun, R. Chen, Y. Zhao, B. Xu and F. Wu, Confined Growth of Nano-Na3V2(PO4)3 in Porous Carbon Framework for High-Rate Na–Ion Storage, ACS Appl. Mater. Interfaces, 2019, 11, 3107–3115 CrossRef CAS.
- W. Ren, Z. Zheng, C. Xu, C. Niu, Q. Wei, Q. An, K. Zhao, M. Yan, M. Qin and L. Mai, Self-sacrificed synthesis of three-dimensional Na3V2(PO4)3 nanofiber network for high-rate sodium-ion full batteries, Nano Energy, 2016, 25, 145–153 CrossRef CAS.
- E. Wang, M. Chen, X. Liu, Y. Liu, H. Guo, Z. Wu, W. Xiang, B. Zhong, X. Guo, S. Chou and S. X. Dou, Organic Cross-Linker Enabling a 3D Porous Skeleton-Supported Na3V2(PO4)3/Carbon Composite for High Power Sodium-Ion Battery Cathode, Small Methods, 2019, 3, 1800169 CrossRef CAS.
- L. Zhao, H. Zhao, X. Long, Z. Li and Z. Du, Superior High-Rate and Ultralong-Lifespan Na3V2(PO4)3@C Cathode by Enhancing the Conductivity Both in Bulk and on Surface, ACS Appl. Mater. Interfaces, 2018, 10, 35963–35971 CrossRef CAS.
- T. Wei, G. Yang and C. Wang, Bottom-up assembly of strongly-coupled Na3V2(PO4)3/C into hierarchically porous hollow nanospheres for high-rate and -stable Na–ion storage, Nano Energy, 2017, 39, 363–370 CrossRef CAS.
- J. Mao, C. Luo, T. Gao, X. Fan and C. Wang, Scalable synthesis of Na3V2(PO4)3/C porous hollow spheres as a cathode for Na–ion batteries, J. Mater. Chem. A, 2015, 3, 10378–10385 RSC.
- C. Li, X. Miao, W. Chu, P. Wu and D. G. Tong, Retracted Article: Hollow amorphous NaFePO4 nanospheres as a high-capacity and high-rate cathode for sodium-ion batteries, J. Mater. Chem. A, 2015, 3, 8265–8271 RSC.
- A. Langrock, Y. Xu, Y. Liu, S. Ehrman, A. Manivannan and C. Wang, Carbon coated hollow Na2FePO4F spheres for Na–ion battery cathodes, J. Power Sources, 2013, 223, 62–67 CrossRef CAS.
- Y. Zhang, S. Guo and H. Xu, Synthesis of uniform hierarchical Na3V1.95Mn0.05(PO4)2F3@C hollow microspheres as a cathode material for sodium-ion batteries, J. Mater. Chem. A, 2018, 6, 4525–4534 RSC.
- R. Ling, S. Cai, D. Xie, W. Shen, X. Hu, Y. Li, S. Hua, Y. Jiang and X. Sun, Double-shelled hollow Na2FePO4F/C spheres cathode for high-performance sodium-ion batteries, J. Mater. Sci., 2017, 53, 2735–2747 CrossRef.
- W. J. Li, C. Han, G. Cheng, S. L. Chou, H. K. Liu and S. X. Dou, Chemical Properties, Structural Properties, and Energy Storage Applications of Prussian Blue Analogues, Small, 2019, 15, 1900470 CrossRef.
- W. Ren, Z. Zhu, M. Qin, S. Chen, X. Yao, Q. Li, X. Xu, Q. Wei, L. Mai and C. Zhao, Prussian White Hierarchical Nanotubes with Surface-Controlled Charge Storage for Sodium-Ion Batteries, Adv. Funct. Mater., 2019, 29, 1806405 CrossRef.
- J. Qian, C. Wu, Y. Cao, Z. Ma, Y. Huang, X. Ai and H. Yang, Sodium-Ion Batteries: Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries, Adv. Energy Mater., 2018, 8, 1870079 CrossRef.
- Q. Xue, L. Li, Y. Huang, R. Huang, F. Wu and R. Chen, Polypyrrole-Modified Prussian Blue Cathode Material for Potassium Ion Batteries via In Situ Polymerization Coating, ACS Appl. Mater. Interfaces, 2019, 11, 22339–22345 CrossRef CAS.
- Y. Jiang, S. Yu, B. Wang, Y. Li, W. Sun, Y. Lu, M. Yan, B. Song and S. Dou, Prussian Blue@C Composite as an Ultrahigh-Rate and Long-Life Sodium-Ion Battery Cathode, Adv. Funct. Mater., 2016, 26, 5315–5321 CrossRef CAS.
- Y. Huang, M. Xie, Z. Wang, Y. Jiang, Y. Yao, S. Li, Z. Li, L. Li, F. Wu and R. Chen, A Chemical Precipitation Method Preparing Hollow-Core–Shell Heterostructures Based on the Prussian Blue Analogs as Cathode for Sodium-Ion Batteries, Small, 2018, 14, 1801246 CrossRef.
- F. Feng, S. Chen, X. Z. Liao and Z. F. Ma, Hierarchical Hollow Prussian Blue Rods Synthesized via Self-Sacrifice Template as Cathode for High Performance Sodium Ion Battery, Small Methods, 2018, 3, 1800259 CrossRef.
- X. Tang, H. Liu, D. Su, P. H. L. Notten and G. Wang, Hierarchical sodium-rich Prussian blue hollow nanospheres as high-performance cathode for sodium-ion batteries, Nano Res., 2018, 11, 3979–3990 CrossRef CAS.
- Y. Sun, S. Guo and H. Zhou, Exploration of Advanced Electrode Materials for Rechargeable Sodium-Ion Batteries, Adv. Energy Mater., 2019, 9, 1800212 CrossRef.
- H. Pan, Y.-S. Hu and L. Chen, Room-temperature stationary sodium-ion batteries for large-scale electric energy storage, Energy Environ. Sci., 2013, 6, 2338–2360 RSC.
- S. Wang, L. Wang, Z. Zhu, Z. Hu, Q. Zhao and J. Chen, All Organic Sodium-Ion Batteries with Na4C8H2O6, Angew. Chem., Int. Ed., 2014, 53, 5892–5896 CrossRef CAS.
- H. Han, H. Lu, X. Jiang, F. Zhong, X. Ai, H. Yang and Y. Cao, Polyaniline hollow nanofibers prepared by controllable sacrifice-template route as high-performance cathode materials for sodium-ion batteries, Electrochim. Acta, 2019, 301, 352–358 CrossRef CAS.
- D. Su, J. Zhang, S. Dou and G. Wang, Polypyrrole hollow nanospheres: stable cathode materials for sodium-ion batteries, Chem. Commun., 2015, 51, 16092–16095 RSC.
| This journal is © the Partner Organisations 2020 |