Spectroscopic studies of the optical properties of carbon dots: recent advances and future prospects
Qingnan
Zhao
,
Wei
Song
 *,
Bing
Zhao
*,
Bing
Zhao
 and
Bai
Yang
and
Bai
Yang

State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun, 130012, P. R. China. E-mail: weisong@jlu.edu.cn
First published on 20th November 2019
Abstract
Carbon dots (CDs) are a new class of luminescent carbon nanomaterials possessing small size, excellent water solubility, low toxicity, tunable light-emitting properties and good biocompatibility, which display bright prospects in catalysis, drug delivery, bioimaging, biosensing and energy conservation and storage. In the past several years, great achievements on the synthetic strategies, characterization techniques, unique optical properties and novel applications of a large variety of CDs have been realized. In this review, the brilliant optical properties of CDs-based nanomaterials via a series of spectroscopic techniques are summarized. After a brief introduction of the characteristic of CDs, we will focus on the optical properties of the CDs, including photoluminescence (PL), near-infrared (NIR) fluorescence, surface-enhanced Raman scattering (SERS), phosphorescence, chemiluminescence (CL), electrochemical luminescence (ECL), and chirality. The correlation between the structure of CDs and their optical properties has been discussed. In addition, some challenges and future prospects in the field of the optical properties of CDs have been highlighted. With the rapid development of their superior optical properties, it is anticipated that CDs will be applicable in a large number of research fields.
1. Introduction
Carbon, one of the richest elements in the universe, usually possesses a number of interesting allotropes. Carbon family materials are mainly composed of fullerenes,1,2 carbon nanotubes,3–5 diamonds,6–8 graphene,9,10 graphdiyne11,12 and carbon dots (CDs).13–15 Among these carbon materials, CDs show the smallest size and superior hydrophilic properties, and exhibit promising applications in catalysis, drug delivery, bio-imaging, bio-sensing and energy devices. CDs were firstly discovered in 2004 by Scrivens and co-workers from a crude suspension during the separation and purification of single-walled carbon nanotubes using a gel electrophoresis method.16 In 2006, Sun and co-workers proposed a chemical synthetic strategy to fabricate luminescent carbon nanoparticles which are called “carbon quantum dots” (CQDs).17 Afterwards, a large number of researchers have devoted their efforts in this field and developed many new synthetic approaches to prepare CDs with varied compositions and chemical structures as well as optical properties. These CDs have shown great promising applications in light-emitting diodes (LEDs), catalysis, sensing, biotechnology, energy devices, etc.18–23 Recently, to avoid energy consumption and environmental pollution, an energy-efficient and sustainable strategy has been developed to prepare green carbon nanodots (CNDs) and yellow graphene quantum dots (GQDs) as well as green CQDs by using different reactants as carbon sources at room temperature, which showed a broad application prospect for bio-imaging, bio-labelling and bio-analysis.24,25Generally, most of the CDs show a size of less than 10 nm and intrinsic fluorescence properties. The chemical structure of CDs is made up of nitrogen/polymeric aggregations/oxygen-based groups (e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, –COOH, C–N, C–O) and sp2/sp3 hybrid conjugated carbon.26–29 Typically, CDs are primarily classified into GQDs, CNDs and CQDs, which present different chemical structures and optical properties, respectively.18,30–32 GQDs are a few layers of graphene sheets with lateral dimensions of less than 10 nm. The zero-dimensional (0D) GQDs converted from two-dimensional (2D) graphene sheets display anisotropism, quantum-size effect and exciton confinement. The conjugated π-domains and the edge structure of the GQDs contribute to their unique luminescence properties. In addition, GQDs usually show favorable crystallinity. Different from GQDs, CNDs are a type of ultrasmall carbon nanoparticles with a spherical morphology and usually an amorphous lattice structure. CNDs are composed of cross-linked or aggregated monomers and linear polymers. However, some CNDs are not entirely linear polymers or disordered monomers owing to the existence of hexagonal carbon networks in amorphous CNDs. CQDs are composed of multiple layers of graphitic sheets and possess a spherical crystalline structure. In addition to typical GQDs, CNDs and CQDs, carbonized polymer dots (CPDs) have also been put forward to verify the vital role of the polymerization and carbonization processes in the synthesis of CDs in recent years.33 So far, a large variety of approaches have been developed to synthesize CDs with different compositions and chemical structures, which are mainly divided into top-down methods based on cutting different carbon materials (e.g., carbon fiber, graphene oxide (GO), fullerenes and graphite electrodes) and bottom-up methods that are generally involved in the organic synthesis of small molecules, self-assembly of polycyclic aromatic hydrocarbons and carbonization of carbohydrates.34–37
O, –OH, –COOH, C–N, C–O) and sp2/sp3 hybrid conjugated carbon.26–29 Typically, CDs are primarily classified into GQDs, CNDs and CQDs, which present different chemical structures and optical properties, respectively.18,30–32 GQDs are a few layers of graphene sheets with lateral dimensions of less than 10 nm. The zero-dimensional (0D) GQDs converted from two-dimensional (2D) graphene sheets display anisotropism, quantum-size effect and exciton confinement. The conjugated π-domains and the edge structure of the GQDs contribute to their unique luminescence properties. In addition, GQDs usually show favorable crystallinity. Different from GQDs, CNDs are a type of ultrasmall carbon nanoparticles with a spherical morphology and usually an amorphous lattice structure. CNDs are composed of cross-linked or aggregated monomers and linear polymers. However, some CNDs are not entirely linear polymers or disordered monomers owing to the existence of hexagonal carbon networks in amorphous CNDs. CQDs are composed of multiple layers of graphitic sheets and possess a spherical crystalline structure. In addition to typical GQDs, CNDs and CQDs, carbonized polymer dots (CPDs) have also been put forward to verify the vital role of the polymerization and carbonization processes in the synthesis of CDs in recent years.33 So far, a large variety of approaches have been developed to synthesize CDs with different compositions and chemical structures, which are mainly divided into top-down methods based on cutting different carbon materials (e.g., carbon fiber, graphene oxide (GO), fullerenes and graphite electrodes) and bottom-up methods that are generally involved in the organic synthesis of small molecules, self-assembly of polycyclic aromatic hydrocarbons and carbonization of carbohydrates.34–37
Many characterization techniques have been employed to evaluate the chemical structure of CDs, such as ultra-violet visible (UV-vis) spectroscopy,38,39,52 Fourier transform infra-red (FTIR) spectroscopy,40,53 X-ray diffraction (XRD),41,47 X-ray photoelectron spectroscopy (XPS),42,53 nuclear magnetic resonance (NMR)43,54 and Raman spectroscopy44,51 (Fig. 1a–i). Typically, UV-vis absorption measurements reveal their main optical absorption in the range of 280–360 nm (Fig. 1a);52 however, the characteristic absorption peaks strongly depend on the preparation methods and the functional groups on the surface of CDs.45,46 XRD technology mainly provides crystallinity structure information of CDs powder,47 which contributes to high-resolution TEM analysis (Fig. 1b). FTIR spectroscopy is also employed to characterize the functional groups on the surface of CDs.48 The typical functional groups of CDs are shown in Fig. 1c, which have been investigated by FTIR spectroscopy.53 XPS is a valuable tool to confirm the elemental composition, electronic state and chemical state of the constituent elements of CDs (Fig. 1d–g).49,53 NMR can be employed to obtain the information about the surface functional groups of CDs (Fig. 1h).50 Raman spectroscopy is able to reveal the ratio of sp2- and sp3-hybrid carbon atoms in the carbon core, which can mainly quantify and characterize the chemical structure of CDs (Fig. 1i).51 In particular, the spectral analysis is critical to evaluate the optical properties of CDs, which is beneficial to understand their luminescence mechanism and optoelectronic applications.
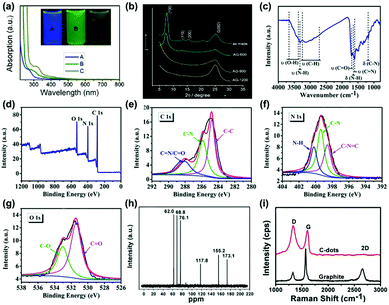 | ||
| Fig. 1 Characterization techniques for the CDs: (a) UV-vis spectra of the CDs synthesized at different reaction temperatures ((A) 120 °C, (B) 100 °C and (C) 80 °C).52 (Reprinted with permission from the American Chemical Society, Copyright 2012.) (b) Wide-angle X-ray diffraction pattern of GQDs synthesized by cyclodehydrogenation of hexaphenylbenzene (as made) and pyrolyzed at different temperatures (600, 900 and 1200 °C).47 (Reprinted with permission from the American Chemical Society, Copyright 2011.) (c) FT-IR spectra of the CDs synthesized from folic acid and ethanol through a hydrothermal reaction and (d) XPS wide scan spectrum of the CDs, (e) C1s, (f) N1s, (g) O1s spectra.53 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2019.) (h) 13C NMR spectra of CDs synthesized through “polymerization” and “carbonization” from ascorbic acid.54 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2012.) (i) Raman spectra (λex = 633 nm) of the high quality CNDs synthesized through a one-step electrochemical approach from graphite rods.51 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2012.) | ||
In the review, we intend to cover the optical characteristics of CDs-based nanomaterials, focusing on the development of the spectroscopic studies of CDs in photoluminescence (PL), near-infrared (NIR) fluorescence, surface-enhanced Raman scattering (SERS), phosphorescence, chemiluminescence (CL), electrochemical luminescence (ECL), and chirality. We will discuss in detail the relationship between the chemical structure and the optical properties of the CDs. Finally, we will give a brief conclusion and future prospects of CDs-based nanomaterials in applications making use of their optical properties. We hope that this review will provide valuable insights to stimulate more people to study the optical mechanism of CDs, and expand further applications of CDs in the fields of biology, catalysis, energy, and environment. Fig. 2 shows the schematic diagram of the main content of the review.
2. Optical properties of CDs
2.1 PL
PL is one of the most fascinating characteristics of CDs. CDs prepared with different approaches and materials show varied compositions and chemical structures, which can emit different colored PL, including deep ultraviolet,55,56 blue,57–59 green,60–62 yellow,63–65 red66–68 and white light emission.69–71 Owing to their unique PL properties, the prepared CDs exhibit outstanding properties in fluorescence calibration, biomedical sensing, and light emitting devices.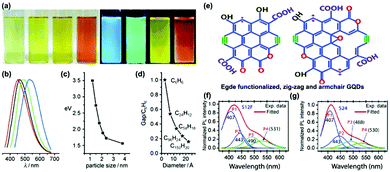 | ||
| Fig. 3 (a) The optical images taken under white (left) and UV light (right) with differently sized CQDs synthesized through a one-step electrochemical approach from graphite rods; (b) PL spectra of differently sized CQDs: the red, black, green, and blue curves correspond to the PL spectra for blue-, green-, yellow-, and red-emission CQDs, respectively; (c) the relationship between the different CQDs sizes and the PL intensities; (d) relationship between the HOMO–LUMO gap and the size of the graphitic fragments.76 (Reprinted with permission from Wiley-VCH Verlag GmbH. Copyright 2010.) (e) The zigzag (magenta dot) and armchair (olive colour bonds) structures of the GQDs. (f) The PL spectra and the Gaussian peak fitting on S12F (12 h reaction grown functionalized GQDs sample). (g) The spectrum of the PL spectra and Gaussian peak fitting on S24 (24 h reaction grown GQDs sample). The peaks P1, P2, P3 and P4 refer to the zigzag and armchair edge states, the COOH/C–OH and CQO/C–O edge functional groups, respectively.81 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2016.) | ||
In addition to the quantum effect, the surface state is another important factor to determine the PL mechanism of CDs. In fact, surface states have not been accurately defined due to the diversity and uncertainty of molecular structures. The surface of CDs has abundant trap states owing to surface oxygen-related groups (–COOH, –OH and C–O–C, etc.), dangling bonds and hybrid carbon atoms of sp2 or sp3. Therefore, surface defect states can occur at any sites except the perfect sp2 domain. For instance, Giri and co-workers synthesized highly fluorescent and edge-controlled GQDs from different solvents.81,82 The edge of CDs mainly contains zigzag and armchair structures (Fig. 3e), providing a specific chemical structure to affect the PL property. By monitoring the PL spectra, the edge state conversion in the GQDs before and after annealing in a H2 and O2 environment has been evaluated. It was found that the PL spectra of GQDs (S12F and S24) can be fitted with four Gaussian peaks (P1-P4). Typically, the blue P1 and P2 peaks are related to the zigzag and armchair edge defects, while the green P3 and P4 peaks are assigned to their edge oxygenated functional groups (Fig. 3f and g).
Liu and co-workers conjectured that an energy transfer process might exist between the carbon core and the surface state. The CDs showed five emission bands at 305, 355, 410, 445, and 500 nm corresponding to the five intrinsic C (4.1 eV), graphitic N (3.5 eV), pyridinic N (3.0 eV), amino N (2.8 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (2.5 eV) related levels, respectively.83 Xiao and co-workers reported that the lifetime discrepancy of PL may not involve the sp2-related states (Fig. 4a and b), but the high temperature made decay dynamics fall sharply due to nonradiative traps of defect state electrons/the sp2 (Fig. 4c and d). The photoexcited electrons via the π–π* transitions included the sp2 energy levels, the defect states and long-wavelength PL (Fig. 4e).84 Yu and co-workers proposed that the PL property of CDs was the result of interactions between isolated pyridine derivative molecules and surface defect states (Fig. 4f). The PL mechanism from the defect emission of CDs was composed of some isolated sp2 clusters, connected pyridine-derivative units, abundant defect states and graphitic cores (Fig. 4g).85 Xiong and co-workers reported eight types of CDs of obvious fluorescence characteristics separated by silica column chromatography with urea and p-phenylenediamine as reaction materials (Fig. 4h–j) and speculated that the PL from CDs surfaces were consisted of bonded oxygen atoms and conjugated carbon atoms.86 The energy gap between the HOMO and LUMO was inversely proportional to the number of incorporated oxygen species. The increment of oxidation degree resulted in a PL redshift (Fig. 4k). Some electrons and excited electrons in the p-orbital might be trapped by the defect state of energy below the p*-orbital, and then return to the holes in the p-orbital. The electrons captured by the surface defect state were eventually relaxed through radiation and non-radiation processes. The PL signal from radiation relaxation was called a defect emission.87
O (2.5 eV) related levels, respectively.83 Xiao and co-workers reported that the lifetime discrepancy of PL may not involve the sp2-related states (Fig. 4a and b), but the high temperature made decay dynamics fall sharply due to nonradiative traps of defect state electrons/the sp2 (Fig. 4c and d). The photoexcited electrons via the π–π* transitions included the sp2 energy levels, the defect states and long-wavelength PL (Fig. 4e).84 Yu and co-workers proposed that the PL property of CDs was the result of interactions between isolated pyridine derivative molecules and surface defect states (Fig. 4f). The PL mechanism from the defect emission of CDs was composed of some isolated sp2 clusters, connected pyridine-derivative units, abundant defect states and graphitic cores (Fig. 4g).85 Xiong and co-workers reported eight types of CDs of obvious fluorescence characteristics separated by silica column chromatography with urea and p-phenylenediamine as reaction materials (Fig. 4h–j) and speculated that the PL from CDs surfaces were consisted of bonded oxygen atoms and conjugated carbon atoms.86 The energy gap between the HOMO and LUMO was inversely proportional to the number of incorporated oxygen species. The increment of oxidation degree resulted in a PL redshift (Fig. 4k). Some electrons and excited electrons in the p-orbital might be trapped by the defect state of energy below the p*-orbital, and then return to the holes in the p-orbital. The electrons captured by the surface defect state were eventually relaxed through radiation and non-radiation processes. The PL signal from radiation relaxation was called a defect emission.87
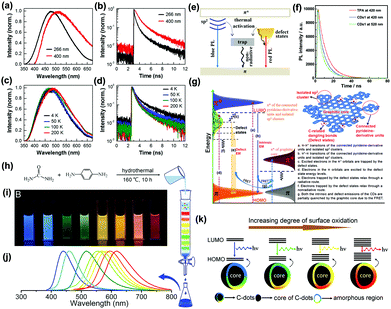 | ||
| Fig. 4 (a) PL spectra of GQDs obtained by thermal deoxidization of GO sheets and (b) PL decay curves of ensemble GQDs excited at 266 and 400 nm by pulsed laser. (c) PL spectra and (d) PL decay curves of ensemble GQDs measured at four temperatures with pulsed laser excitation at 266 nm. (e) Energy level structures on GQDs; energy level structures to explain the optical behaviors of photoexcited electrons in both GO sheets and GQDs.84 (Reprinted with permission from the American Chemical Society, Copyright 2013.) (f) PL decays of free TPA (3,5-dihydro-5-oxo-2H-thiazolo[3,2-a]pyridine-3,7-dicarboxylic acid) and CDs synthesized by thermal treating a mixture of citric acid and amino group-containing molecules at 345 nm laser excitation. (g) Representation of the PL mechanism of CDs.85 (Reprinted with permission from Elsevier Ltd, Copyright 2017.) (h) One-pot synthesis and purification route for CDs with distinct fluorescence characteristics. (i) Eight CDs samples under 365 nm UV light. (j) Corresponding PL emission spectra of the eight samples, with maxima at 440, 458, 517, 553, 566, 580, 594, and 625 nm. (k) Model for the tunable PL of CDs with different degrees of oxidation.86 (Reprinted with permission from the American Chemical Society, Copyright 2016.). | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, amino N and pyridine N groups, respectively. It could be concluded that the narrow band gaps of surface states were important for optical properties of CDs.
O, amino N and pyridine N groups, respectively. It could be concluded that the narrow band gaps of surface states were important for optical properties of CDs.
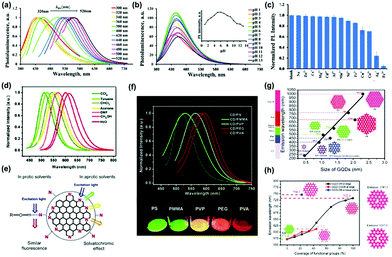 | ||
| Fig. 5 (a) The excitation-dependent normalized PL for NS-CDs via a one-step facile microwave-assisted pyrolysis using citric acid and thiourea, (b) pH-dependent PL spectra at different pH values, (c) the normalized PL intensity of NS-CDs in different metal ions.88 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2019.) (d) The effect of solvents on the PL of CDs prepared from p-phenylenediamine excited by 420 nm light. (e) The emission processes of the CDs in different solvents. (f) PL spectra of five CDs/polymer films (excited at 420 nm).89 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2017.) (g) Calculated emission wavelength (nm) of the diameter of GQDs using the time-dependent density-functional theory (TDDFT) method. The solid line is the linear fit of zigzag-edged GQDs. (h) Emission wavelength versus oxidized GQDs attached –OH and –COOH groups to edge carbon atoms. (coverage of –OH and –COOH groups from 0 to 100%).90 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2014.) | ||
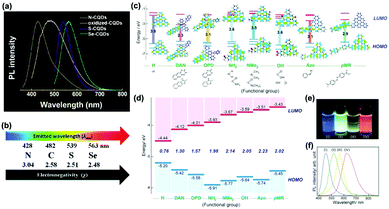 | ||
| Fig. 6 (a) Normalized PL spectra of oxidized-CQDs and heteroatom (N, S, Se) doped CQDs, and (b) the relationship between the electronegativity of heteroatoms and the emission wavelength of doped CQDs.95 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2014.) (c) Predicted energy level diagrams for nitrogen-functionalized GQDs with different functional groups using ab initio calculations by the Gaussian 09 package. (d) Measured energy level diagram for nitrogen-functionalized GQDs. (e) PL image of nitrogen-functionalized GQDs in aqueous solution excited by a UV lamp (365 nm). (f) Normalized PL spectra (excited at 380 nm) of aqueous nitrogen-functionalized GQDs.97 (Reprinted with permission from Wiley-VCH Verlag GmbH, Copyright 2016.). | ||
The quantum yield (QY) is defined as the number of emitted photons proportional to the number of absorbed photons. The QY of naked CDs is rather low (2% to 29.9%) because of epoxide and carboxylic groups acting as non-radiative electron–hole recombination centers, which could be determined by the fluorescence spectrum technique. Therefore, many strategies have been reported to improve the QY by changing some factors, such as the synthesis temperature,98 element doping,99 metal-enhanced fluorescence100 and surface passivation/modification.101 Among these, element doping is one of the most efficient routes to achieve high QY. For example, Li and co-workers prepared manganese doped CDs, possessing a quantum yield of 54%, which could be used to detect heavy metallic Hg2+ with a detection limit level of nM.102 Yang and co-workers synthesized nitrogen/silicon co-doped CQDs (N, Si-CQDs) with a high QY of 97.3% through a hydrothermal approach, which had great potential to be applied in white LEDs.103
The PL decay lifetime reflects the time of the electrons in the excited state before going back to the ground state, which plays a vital role in practical applications in sensing, optoelectronics and bio-imaging.104 The PL decay lifetime is usually influenced by the concentration of reporter, excitation laser power and even temperature conditions. For example, Zbořil and co-workers prepared N,S-co-doped CDs (N,S-CDs) and found that the PL lifetime reduced from 11.0 to 5.3 ns with the temperature increasing from 2 to 80 °C.105 This result demonstrated the potential applications of the N,S-CDs for thermal sensing.
In a word, the unique PL properties of varied types of CDs have been investigated in recent years. It is of great importance to reveal the PL mechanism from steady PL spectroscopy. In addition, the unique chemical and electronic structure of CDs contributes to the high QY and long PL decay lifetime. However, some new spectroscopic measurements are necessary to clarify the PL properties of CDs.
2.2 NIR fluorescence
So far, scientists have taken great efforts to synthesize various types of CDs with different strategies. The optimal excitation wavelength of most CDs is in the UV range, which limits their broad applications in biomedical fields. On the other hand, near-infrared emissive CDs (wavelength >650 nm) can decrease autofluorescence and penetrate a bio-sample. Therefore, NIR emitting CDs are very suitable for optical bio-imaging. For example, Hao, Zeng and co-workers developed novel NIR-II-emitting CDs excited with a 808 nm laser, which possessed a high quantum yield at 900–1200 nm and excellent biocompatibility. Furthermore, the prepared CDs showed high photothermal efficiency (30.6%), and were successfully applicable in in vivo NIR-II bioimaging (Fig. 7a).106 The results showed that the tumor after intratumoral injection (i.v.) and intravenous injection (i.t.) of CDs disappeared on the sixth day compared with injection of PBS solution (Fig. 7b–e).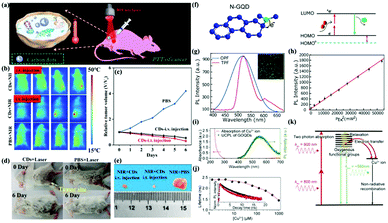 | ||
| Fig. 7 (a) Schematic diagram of photothermal therapy of cancer in vivo using NIR-II emission of CDs. (b) Representative infrared thermal images of tumor-bearing mice under 808 nm laser irradiation. (c) Time course change in the relative volume after treatment by using CDs and PBS. (d) Digital photographs of the tumor-bearing mice after different treatments. (e) Photographs of tumors harvested from mice after 6 days of treatment.106 (Reprinted with permission from the American Chemical Society, Copyright 2019.) (f) Fluorescence mechanism between a π-conjugated system and N-GQDs. (g) Two-photon-induced fluorescence spectrum of N-GQDs solution under 800 nm femtosecond laser excitation. (h) Quadratic relationship of the fluorescence intensity of the N-GQDs aqueous solution with different excitation laser powers at 800 nm.110 (Reprinted with permission from the American Chemical Society, Copyright 2013.) (i) UV-vis absorption spectrum of Cu2+ ions and the PL spectrum of GOQDs excited by a wavelength of 800 nm. (j) PL lifetime of the GOQDs depending on the Cu2+ concentration. (k) GOQD quenching modeling via electron transfer from the UCPL GOQDs to the Cu2+ ions.113 (Reprinted with permission from Elsevier Ltd, Copyright 2014.) | ||
Recently, Qu, Rogach and co-workers reported CDs with remarkable two-photon-induced NIR emission as well as three-photon-induced red emission in dimethyl sulfoxide.107 The sulfoxide/carbonyl connected to the edges and the outer side of the CDs led to an increase of the discrete energy levels and degree of surface oxidation. Moreover, the functionalized CDs promoted the NIR absorption band and enhanced NIR luminescence, leading to their potential applications in NIR fluorescence imaging. Recently, Wu, Xiao and co-workers synthesized N-doped CDs through a solvothermal reaction, exhibiting NIR emission with two photon luminescence.108 In addition, the prepared N-doped CDs displayed a piezochromic luminescence with reversibility, showing a red- and blue-shifted PL with applied pressure increasing from 0.07 to 1.70 GPa and a red- and blue-shifted PL with an external pressure from 5.18 GPa to 1 atm, which could expand their applications to detect external pressure stimuli.
Upconversion PL is an anti-Stokes type emission which exhibits emission light of shorter wavelength (single photon of higher energy) through absorption of two or more photons of longer wavelength. Then two- or multi-photon fluorescence properties can be achieved in the near-infrared region, demonstrating promising applications in the biomedical imaging field. In 2007, Sun and co-workers first reported up-converted CDs with two-photon fluorescence, which was excited using an 800 nm femtosecond infrared pulsed laser.109 From then on, many researchers have focused on this field. For example, N-doped GQDs (N-GQDs) have been synthesized from GO in dimethylformamide solution via a solvothermal reaction, showing excellent two-photon fluorescence properties due to the rigid plane and large π-conjugated system of N-GQDs (Fig. 7f). Fig. 7g (the inset is a fluorescence image of N-GQDs) shows the fluorescence spectrum of N-GQDs solution induced by an 800 nm wavelength femtosecond laser.110 The PL intensity had a quadratic relationship with the excited laser power (Fig. 7h). It was found that two-photon fluorescence with a much narrower bandwidth than the one-photon fluorescence spectrum was obtained. Photon absorption with NIR excitation contributes to the green fluorescence of the prepared N-GQDs. Cui and co-workers demonstrated a facile strategy to enhance the upconversion PL and tailor the red-shift fluorescence of CDs via the introduction of H2O2 during a hydrothermal reaction.111 It could be considered that the upconverted PL property resulted from a multiphoton active process.
The upconverted PL property enabled CDs promising applications in sensing, catalysis, and bio-imaging fields. Recently, highly fluorescent N-doped CDs have been prepared through a solar irradiation strategy between o-phenylenediamine and citric acid, exhibiting not only ultra-violet (378 nm) pumped emissions but also up-converted PL signals in the NIR range (750 nm).112 The prepared N-doped CDs provides an efficient multi-modal sensing platform for Hg2+. Cho, Seo and co-workers explored quenching properties of upconversion PL on graphene oxide quantum dots (GOQDs) and Cu2+ ions (Fig. 7i–k).113 It was found that the PL quenching was attributed to an electron transfer instead of an energy transfer mechanism. In recent years, up-converted CDs-based hybrids have aroused a lot of attention for their promising applications in photocatalysis and cell imaging.114–117 For instance, the photocatalytic activity of rutile TiO2/GQDs hybrids for methylene blue degradation under visible light was significantly improved compared with individual rutile TiO2 nanoparticles and bare GQDs. Furthermore, the photocatalytic activity of the rutile TiO2/GQDs was also higher than that of anatase TiO2/GQDs. The superior catalytic efficiency of the rutile TiO2/GQDs was owing to the upconverted PL peak of GQDs (407 nm) under visible light irradiation with an energy (3.05 eV) larger than the band gap of rutile TiO2 (3.0 eV) but smaller than that of anatase TiO2 (3.2 eV).114 Another example of novel CQDs modified Bi20TiO32 showed a rate constant 4.3 times higher than that of pure Bi20TiO32 for the photodegradation of isoproturon, which was attributed to the function of CQDs as an up-conversion photosensitizer.115 Recently, CDs-based hybrids have also been designed for two-photon biosensing applications. Tian and co-workers for the first time reported the preparation of a CDs-based inorganic–organic hybrid system for imaging and biosensing of pH in tumor tissue, e.g. mouse LLC-MK2 cells and human A549 cells at a depth of 65–185 μm through two-photon microscopy, demonstrating the practical applications of CDs in living cells.116
2.3 SERS spectroscopy
SERS is a non-invasive spectroscopic technique based on molecular vibrational fingerprints that quantifies and identifies ultra-trace analytes.118,119 Generally, plasmonic nanostructures composed of Ag, Au, Cu and Al have been employed as SERS substrates to boost Raman signals, which originates from two mechanisms: (1) electromagnetism (EM) based on surface plasmon resonance (SPR) on the metal surface,120 (2) charge transfer (CT) prompted by the interaction between the metal surface and adsorbed molecules.121 The development of SERS is associated with progress of the fabrication of novel substrates. Thus, the manufacture of efficient SERS substrates becomes more vital in this study field. The “hot spots” existing in the nanogap between metal nanoparticles is considered to be a key issue to produce outstanding SERS substrates.122 CDs without doping and modification were first regarded as SERS substrates by Shi and co-workers in 2012.123 An electrophoresis deposition strategy was developed to assemble GQDs into nanotube arrays by using a nanoporous anodic aluminum oxide membrane as a template. By using Rhodamine 6G (R6G) molecules as probes, the highest 74-fold SERS enhancement could be obtained on the surface of GQDs nanotubes, which was much higher than those on the graphene substrate. The SERS spectra revealed that the detection limit could reach around 10−9 M, demonstrating their high sensitivity. In addition to dye probes, this SERS substrate has also shown the capacity for 2,4-dinitrotoluene (2,4-DNT) detection. The SERS spectrum showed similar vibrational frequencies to those previously reported Au and Ag substrates. Similar to the graphene film, efficient CT between GQDs and target molecules should contribute to the excellent SERS property of the GQDs nanotubes. Furthermore, the unique porous structure of the GQDs nanotubes with a rough surface guaranteed the large specific surface area for target molecule adsorption as well as incident light capture, which is another key factor to generate unique SERS activity. This fact could be verified by the weak SERS signals on the surface of GQDs microbowls.Recently, CDs have been reported to show strong catalysis to generate gold and silver nanomaterials as efficient SERS substrates for quantitative analysis.124–126 In addition, CDs have also been used to support Au and Ag materials for efficient SERS substrates to enhance their SERS activity. For example, Qu and co-workers fabricated a honeycomb architecture composed of CQDs to support gold nanoparticles as a SERS substrate, exhibiting 8–11 times higher SERS activity than the traditional Au nanoparticle SERS substrate. It was speculated that the CQDs honeycomb structure and the uniformly distributed small Au particle size (<10 nm) contributed to the improved Raman response.127 Recently, our group has synthesized Ag@CDs nanoparticles (NPs) with a core–shell structure serving as SERS substrates.128 It was found that the Ag@CDs NPs could quench the fluorescence of CDs through the introduction of Ag, which was beneficial for the SERS enhancement. In addition, the superior adsorption of target molecules by Ag@CDs also contributed to their SERS enhancement. By using p-aminothiophenol (PATP) as target molecules, the typical a1 vibration mode at 1077 cm−1 and b2 vibration modes at 1143, 1390, 1434, and 1570 cm−1 were observed. The enhancement factor value was calculated to be about 6.7 × 105 for the Ag@CDs NP substrate. Furthermore, the prepared Ag@CDs NPs showed a better SERS enhancement than individual Ag NPs, demonstrating the synergistic effect between Ag and CDs for the SERS activity. More interestingly, the core–shell Ag@CDs NPs also exhibited excellent enzymatic catalytic activity towards the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2 (Fig. 8a), plasmon driven p-nitrothiophenol (PNTP) dimerization into 4,4′-dimercaptoazobenzene (DMAB) (Fig. 8b), and the catalytic reduction of PNTP to PATP by NaBH4 (Fig. 8c). Similarly, we have also prepared a Ag NPs/CDs hybrid via a simple chemical coupling reaction strategy as a SERS substrate to study the enhancement mechanism.129Fig. 8d illustrates the relationship between the peak intensity ratio (1144 (b2), 1075 (a1) cm−1) of PATP molecules and the excitation energy on the surface of Ag NPs/CDs and Ag NPs. It could be concluded that the SERS signal of Ag NPs was associated with the polarizability of the molecules and the change of the potential under higher excitation energy, which was consistent with a previous report.130 The SERS signal of Ag NPs/CDs was related to the degree of CT. Meanwhile, the relationship between the relative intensity (b2/a1) and the laser power of the two bands was investigated (Fig. 8e and f), from which it could be concluded that the SERS chemical enhancement mechanism was mainly controlled by the enhancement of the b2 band.
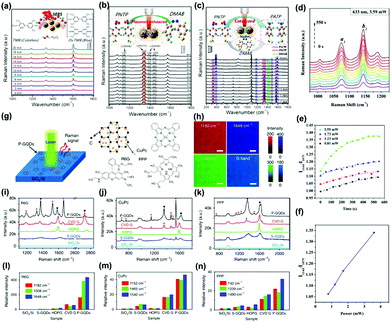 | ||
| Fig. 8 Time-dependent SERS spectra of the catalytic reaction by core–shell Ag@CDs NPs. (a) The oxidation of TMB by H2O2 (3 × 10−3 M), (b) plasmon-driven catalytic reaction of PNTP dimerizing into DMAB, and (c) catalyzed reduction of PNTP to PATP.128 (Reprinted with permission from the American Chemical Society, Copyright 2016.) (d) The Raman intensity of PATP molecules on Ag NPs/CDs versus time, (e) the SERS intensity ratio (I1144/I1075) at different times with different powers, (f) the SERS intensity ratio (I1144/I1075) versus power at 300 s.129 (Reprinted with permission from Elsevier B.V. Copyright 2017.) (g) Schematic of a P-GQDs substrate for Raman measurement and (h) Raman mapping of the intensity of the G band and the characteristic peaks of R6G on P-GQDs. (i–n) Raman spectra of thermally evaporated Rhodamine 6G (R6G), copper phthalocyanine (CuPc) and Protoporphyrin IX (PPP) on SiO2/Si, GQDs produced by solution processes (S-GQDs), highly oriented pyrolytic graphite (HOPG), transferred chemical vapor deposition (CVD) graphene and P-GQDs, respectively. (e–g) Relative intensity of the Raman signals of R6G, CuPc and PPP on different substrates, normalized to the signals on SiO2/Si.134 (Reprinted with permission from Nature Publishing Group, Copyright 2018.) | ||
In addition to Au and Ag hybrids with CDs,131,132 a Cu/CDs hybrid has also been prepared to be an efficient SERS substrate.133 For example, Jiang and co-workers reported the fabrication of Cu/CDs assemblies with varied morphologies through an organic–inorganic interface interaction. Owing to the lattice-matching, CDs selectively deposited on the Cu{111} plane to form a unique Cu/CDs hybrid. Further, CDs in the Cu/CDs hybrid not only prevented Cu oxidation but also provided a unique interface for optical conversion and CT, contributing to the enhanced SERS effect. The SERS detection limit reached 10−8 M by using PATP as target molecules. The SERS enhancement mechanism of GQDs using R6G molecules as probes has been investigated by an ab initio density functional theory (DFT) calculation approach.134 The CT process between GQDs and R6G would occur when their HOMO levels were close, which contributed to the SERS signal. In addition, the authors also investigated the SERS substrate based on the quasi-equilibrium plasma-enhanced chemical vapor deposition GQDs (P-GQDs) grown on SiO2/Si (Fig. 8g). P-GQDs as the SERS substrate was demonstrated to be reliable via analyzing the Raman spectra (Fig. 8i–k) and relative intensity (Fig. 8l–n) of R6G, copper phthalocyanine (CuPc) and Protoporphyrin IX (PPP) on different substrates, with an ultra-low detection concentration (1 × 10−9 mol L−1) for R6G. The authors conjectured that the superior SERS activity could be attributed to the high-quality GQDs with atomically clean surfaces and a large number of edges, as well as the enhanced CT between molecules and GQDs with appropriate diameters due to the existence of Van Hove singularities in the electronic density of states.134
2.4 Phosphorescence
Phosphorescence refers to a radiative transition and spin-forbidden process from the triplet state (T1) to the singlet ground state (S0). The phosphorescence lifetime is longer than the fluorescence lifetime.135 As an emerging optical response material, the room temperature phosphorescent properties of CDs have been investigated, which could be monitored by steady state PL spectroscopy and time-resolved phosphorescence spectroscopy. The first observation of phosphorescence from CDs in a polymer matrix was reported by Zhao and co-workers.136 Yang and co-workers concluded that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds facilitate the production of triplet excitons (Fig. 9a).137 Moreover, the complex matrix was a key factor in preventing the triplet state from a long lifetime quenched by inhibiting the nonradiative processes (Fig. 9b–e). It can be seen from the optical spectra of N doped CDs incorporated into biuret composite matrices that they possessed a good oxygen barrier performance and the hydrogen bonds were effective in suppressing vibrational dissipation at all temperatures (Fig. 9f–i). In addition, the N doped CDs-based composite with distinguishable lifetime codes was expected to be applied in data encryption (Fig. 9j and k). The steady-state PL and phosphorescence spectra presented the contributions of hydrogen bonding between N-doped CDs and biuret to the emissive triplet relaxation. The phosphorescence mechanism is ascribed to the generation of novel energy level structures from C
N bonds facilitate the production of triplet excitons (Fig. 9a).137 Moreover, the complex matrix was a key factor in preventing the triplet state from a long lifetime quenched by inhibiting the nonradiative processes (Fig. 9b–e). It can be seen from the optical spectra of N doped CDs incorporated into biuret composite matrices that they possessed a good oxygen barrier performance and the hydrogen bonds were effective in suppressing vibrational dissipation at all temperatures (Fig. 9f–i). In addition, the N doped CDs-based composite with distinguishable lifetime codes was expected to be applied in data encryption (Fig. 9j and k). The steady-state PL and phosphorescence spectra presented the contributions of hydrogen bonding between N-doped CDs and biuret to the emissive triplet relaxation. The phosphorescence mechanism is ascribed to the generation of novel energy level structures from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds on the surface of the N-doped CDs. Moreover, an ultralong phosphorescence lifetime of 1.06 s was achieved for the prepared N-doped CDs encapsulated in biuret under 280 nm excitation.
N bonds on the surface of the N-doped CDs. Moreover, an ultralong phosphorescence lifetime of 1.06 s was achieved for the prepared N-doped CDs encapsulated in biuret under 280 nm excitation.
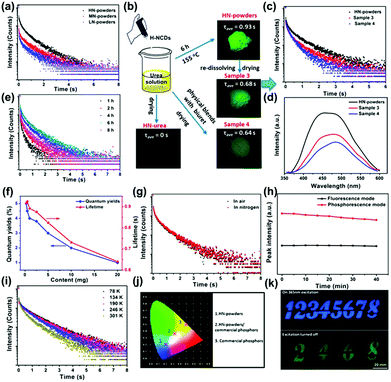 | ||
| Fig. 9 (a) Lifetime decay profiles of the N-doped CDs in composite matrices, and the emission band at 490 nm excited at 280 nm. (b) Flow diagram of verification experiments. (c) Lifetime decay profiles of the emission band. (d) Phosphorescence spectra of sample powders. (e) Lifetime decay profiles of the emission band. (f) Average lifetimes and phosphorescence quantum yield (PQYs). (g) Lifetime decay profiles. (h) Repeated excitation illumination at 280 nm for 40 min. (i) Lifetime decay profiles of the emission at 490 nm. (j) Emission color coordinates of assembled LEDs. (k) Security protection applications.137 (Reprinted with permission from the American Chemical Society, Copyright 2016.) | ||
Organic molecules have also been used to construct hydrogen-bonded networks with CDs by utilizing water molecules to promote phosphorescence emission.138 The prepared CDs–cyanuric acid (CA) hybrid displayed a high phosphorescence lifetime of 687 ms, which was ascribed to the enhanced rigidity of the CDs after the introduction of CA. The enhancement mechanism for the phosphorescence emission of the CDs/CA system has also been investigated.139 There were two main aspects that were involved in the reducing the surface defect and increasing the degree of conjugation between the carbon core and carboxyl groups. Zhou and co-workers proposed a strategy to effectively enhance phosphorescence signals by using water molecules to build hydrogen bond networks between cyanuric acid and CDs, which effectively stiffens the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the CDs, as well as greatly increases the rigidity of the overall system.140
O bond of the CDs, as well as greatly increases the rigidity of the overall system.140
In the last few years, phosphorescence energy transfer systems between CDs and inorganic matrixes have attracted increasing attention for their promising applications for sensing and security applications. For example, phosphorescence energy transfer has been observed to take place between carbon nanotubes and Mn-doped ZnS quantum dots conjugated with capture ssDNA, which could be used for supersensitive detection of DNA.141 Similarly, the phosphorescence efficiency of CDs has been enhanced by the introduction of an inorganic layered double hydroxide (LDH) matrix, which improved the rate of radiative attenuation from T1 to S0 and intersystem crossing (ISC) from S1 to Tn.142 A molten salt (MS) strategy has been used to prepare CDs@MS, showing an excitation dependent phosphorescence property.143 The phosphorescence generation could be related to the preservation of the triplet state of CDs by the rigid structure from the crystallization of MS. The high charge density of metal ions reduced the energy gap of CDs for the achievement of ISC. It is well known that boron is an electron withdrawing atom, which is able to reduce the energy gap between the singlet and triplet states. Therefore, the introduction of boric acid into CDs was beneficial for the ISC between S1 and T1, which enhanced the triple excitons and the phosphorescence emission.144 The prepared heteroatom-free-CDs/boric acid hybrid displayed a yellow-green phosphorescence and an ultrahigh lifetime of 1.6 s.
In addition to the polymers, organic molecules and functional inorganic matrices, individual CDs have also been observed to show room temperature phosphorescence.145–149 For example, Lin and co-workers proposed a self-immobilization strategy to prepare N and P doped fluorescence emissive CDs. After a further heating process, the product can convert fluorescence to ultralong phosphorescence.145 An excitation-dependent phosphorescence spectrum of the CDs powder was achieved. The steady state fluorescence and afterglow decay spectra revealed an average lifetime of about 1.39 s. It could be concluded that the formed compact cores via a further heating step self-immobilize the excited triplet excitons due to the intraparticle hydrogen bonds. Furthermore, the N and P dopants are also beneficial to form an n → π* transition to improve the ISC process for populating triplet species. Similarly, a microwave irradiation approach has also been demonstrated to fabricate N and P doped-CDs, displaying the longest phosphorescence lifetime and undergoing an n–p* transition to effectively fill triplet excitons.146 F and N co-doped CDs (FNCDs) have also been prepared to emit green self-protective phosphorescence with longevous triplet excited states.147 The phosphorescence of the FNCDs results from the n–π* electron transitions for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–N bonds as well as the reduction of the phosphorescence quenching to oxygen by the hydrogen bonds between F and N–H. Recently, Yang and co-workers demonstrated that a novel covalently crosslinked framework structure of the polymeric CDs could efficiently suppress the nonradiative transitions and the abundant energy levels of the polymeric CDs increased the ISC.148 Therefore, a phosphorescence emission with a broad peak centering at 494 nm was observed for the prepared polymeric CDs. In addition, the lifetime of the obtained polymeric CDs was calculated to be about 0.03 s. The crosslink enhanced emission effect has been regarded as the key factor for the phosphorescence production.
N/C–N bonds as well as the reduction of the phosphorescence quenching to oxygen by the hydrogen bonds between F and N–H. Recently, Yang and co-workers demonstrated that a novel covalently crosslinked framework structure of the polymeric CDs could efficiently suppress the nonradiative transitions and the abundant energy levels of the polymeric CDs increased the ISC.148 Therefore, a phosphorescence emission with a broad peak centering at 494 nm was observed for the prepared polymeric CDs. In addition, the lifetime of the obtained polymeric CDs was calculated to be about 0.03 s. The crosslink enhanced emission effect has been regarded as the key factor for the phosphorescence production.
2.5 CL
CL can be described as a process of emitting photons from the excited states to the ground states during a chemical reaction.150,151 Most properties of CL are similar to fluorescence. The difference is that the excitation energy of fluorescence comes from the excitation of external light sources, while the excitation energy of CL is produced by self-chemical reactions. In a chemical reaction, a molecule or an atom is excited by absorbing the chemical energy generated during the reaction. The energy is released by means of optical radiation when the excited molecule or atom returns to the ground state. The remarkable property of CL usually shows a high sensitivity due to the absence of an external light source. At present, the work on CL analysis based on CDs is still in its infancy.Lin and co-workers first studied the CL properties of CDs in a mixed solution of NaNO2 and H2O2, which were the main emitters in the CDs–NaNO2–H2O2 system.152 The CL spectra showed that the systems of NaNO2 + H2O2 and CDs + H2O2 only exhibited a weak CL emission, while the system of NaNO2 + H2O2 + CDs produced a very strong CL. This result implied that the product (oxidant, ONOOH and OH3) during the reaction of nitrites and acidified H2O2 could transform and inject small holes of the CDs. This process, accelerating the hole-injected annihilation and increasing the number of injected electrons, brought about the release of energy in the form of CL emissions.153–156 Lin and co-workers also developed a CL platform based on N-doped CDs to improve the CL sensitivity in a KIO4–H2O2 system in a basic medium (Fig. 10b).157 As shown in Fig. 10a and c, the intensity (3) was much higher compared with (1) and (2) because of the decomposition of H2O2 and energy transfer of 1O2 as emissive species. Dong and co-workers reported a CNDs/K2S2O8 system with enhanced CL efficiency induced by triethylamine (TEA) (Fig. 10f).158 The CL spectrum showed that the CL intensity of the CDs/K2S2O8 system increased after the introduction of TEA (Fig. 10d). The enhanced CL emission could result from the reduction of CNDs by the TEA free radicals to generate excited CNDs. Furthermore, the addition of CNDs at the end is beneficial to increase the CL signal (Fig. 10e). Similar to the CNDs, Abdolmohammad-Zadeh and co-workers constructed a graphitic carbon nitride quantum dots (g-CNQDs)–K3Fe(CN)6 CL system to achieve enhanced CL emission.159 After mixing the g-CNQDs and K3Fe(CN)6, a wide peak centered at around 503 nm was observed, which was similar to but significantly higher than the fluorescence band of g-CNQDs, revealing the efficient CL property of the g-CNQDs–K3Fe(CN)6 system. Due to CL quenching in the presence of Hg(II) ions binding with the g-CNQDs and the CT process, this system could be used to detect Hg(II) with a high sensitivity and excellent selectivity. Recently, Amjadi and co-workers prepared Si doped CDs (Si-CDs) to construct a novel Si-CDs–Fe(II)–K2S2O8 CL system, showing a CL emission peak at around 500 nm.160 During the reaction, Si-CDs were used as receptors and 1(O2)2* was used as a donor, producing excited-state Si-CDs with CL emission. Besides, the group illuminated the CL system based on a CNQDs–Cu(II)–H2O2 system, which took place under the condition of electron and hole injection (oxygen radicals).161 This CL reaction has been utilized for the sensitive detection of H2O2 and glucose. Similarly, N-(aminobutyl)-N-(ethylisoluminol)-GQDs–H2O2,162 Cu2+-CDs,163 and Cu(II)/Cu2O/N-doped GQDs–luminol–H2O2 CL systems164 have also been constructed to show superior CL properties for biosensing applications. It has been reported that the surface groups of the CDs play an important role in their CL properties. For instance, the CL intensity of CDs prepared in peroxynitrite was related to the concentration of C–O group-related O-states, demonstrating that ample C–O functional groups in CDs with high O-states could promote the electron transfer.165 A recent report showed that N-doped GQDs strongly strengthen the CL intensity of the permanganate-sulfite system. However, the enhancement mechanism for N-doped GQDs was not dependent on the particle size.166 The CL spectra revealed that pyridinic N in N-GQDs acted as a catalytic active site to the amplified CL emission. With the increment of the pyridinic N concentration, an enhanced CL intensity was obtained.
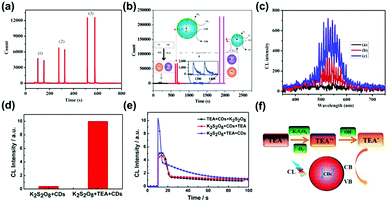 | ||
| Fig. 10 (a) CL curves of a KIO4–H2O2–N-doped CDs system ((1) H2O2 was added to the mixture of KIO4 and N-CDs, (2) N-CDs was added to the mixture of KIO4 and H2O2, (3) KIO4 was added to the mixture of H2O2 and N-CDs). (b) The mechanism of enhancement of the CL of the KIO4–H2O2 system by N-doped CDs in acidic (the decreasing effect) and basic (increasing effect) solutions. (c) CL spectra of the KIO4–H2O2 system in the presence and absence of N-doped CDs and NaOH (a: KIO4–H2O2, b: KIO4–H2O2–N-doped CDs, c: KIO4–H2O2–N-doped CDs–NaOH).157 (Reprinted with permission from the American Chemical Society, Copyright 2016.) (d) The CL spectrum of the CNDs/K2S2O8 system and the CNDs/TEA/K2S2O8 system. (e) CL spectrum of the CNDs/TEA/K2S2O8 system under different reagent mixing orders. (f) The scheme of the CL mechanism of the CNDs/K2S2O8/TEA system.158 (Reprinted with permission from the American Chemical Society, Copyright 2016.) | ||
2.6 ECL
ECL is a phenomenon where chemical substances that are produced on the electrode surface go through electron transfer reactions to realize excited states to generate light emission.167,168 ECL mechanisms are categorized as two types according to the source of free radicals, which are the annihilation pathway and the co-reaction pathway, respectively. ECL techniques based on the CDs materials are widely used because of their simple synthesis, low-cost equipment requirement, high sensitivity and a wide linear range. The first investigation of CDs on ECL properties was reported by Yang and co-workers in 2009.169 The CNDs were prepared through a simple microwave reaction by using poly(ethylene glycol) (PEG-200) and saccharide as the carbon sources. The ECL spectrum of the CNDs showed a similar behavior to those of traditional CdSe, CdTe, and Si nanocrystals. The ECL mechanism should be ascribed to the transfer of the excited CNDs (R*) that are formed from the electron-transfer annihilation of the oxidized state (R˙+) and the reduced state (R˙−) under a potential cycle to the ground state via a radiative pathway, which efficiently emitted a photon. Similar to CNDs, GQDs have also been proved to be a brilliant ECL material.170 It has been reported that greenish-yellow colored GQDs were prepared from a microwave reaction via cleaving GO by acids, exhibiting a maximum wavelength at around 512 nm in the ECL spectrum. Most importantly, the high content of sp2 carbon domains in GQDs provides their unique energy gap compared with graphene, which is beneficial for the electron transport during the ECL process. CQDs could also be assembled into nanospheres with the assistance of a surfactant (pluronic F127), showing an excellent ECL stimulus response in water, while a weak ECL signal in a nonpolar solvent.171 This is because the nonpolar CQDs (NCQDs)/F127 hybrid possesses two secondary structures, e.g., a hydrophobic layer from the polyoxypropylene (PPO) moiety of F127 and NCQDs, and a hydrophilic layer from the PPO moiety of F127. In aqueous solution, the hydrophilic layer could adsorb SO4˙− to excite nearby NCQDs, producing superior ECL emission. However, in a nonpolar solvent, the NCQDs are separated from F127, which are difficult to excite by the SO4˙−, generating weak ECL signals.High conductive materials have been utilized to improve the ECL property as a conducting bridge for efficient electron transfer. For example, a hybrid made of sandwiched CDs labeled on secondary antibodies on a glass carbon electrode showed certain ECL signals.172 After the introduction of graphene as a conducting bridge, about 30-fold ECL enhancement was achieved. In addition to graphene, Au nanoparticles have also been used as signal transduction units for ECL amplification. Qiu and co-workers proposed a dual-potential ECL ratiometric approach to produce ECL signals by using GQDs and luminol as cathodic and anodic ECL emitters, and Au NPs as signal transduction units.173 The introduction of Au nanoparticles could both reduce the cathodic ECL of GQDs and increase the anodic ECL of luminol, demonstrating a dual-potential ECL ratiometric strategy to monitor kinase activity.
The ECL technique based on CDs could be used for sensing applications with a high sensitivity. For example, Wang and co-workers reported a dual-peak ECL system to detect iron ions, showing a higher efficiency than single peak ECL.174 In this system, the ECL system at −2.84 V (ECL-1) was induced through an electron transfer reaction between the reduced nanoparticles and co-reactant, while the ECL system at −1.71 V (ECL-2) resulted from the quenching process. In addition to iron ions, a sensitive ECL biosensor based on N-doped CDs-Pd@Au hexoctahedra has been constructed for the detection of intracellular Pb2+ with a detection limit of 0.33 ng mL−1 and relative standard deviations (RSDs) of no more than 5%.175 Recently, a solid-state ECL sensor (CDs-Fe(CN)63−/4−) with an excellent signal amplification has also been constructed, which could be used to detect glutathione with a low detection limit of 54.3 nM and excellent selectivity.176 Another example of a silica-doped GQDs-based ECL system with fluorescence was reported to detect ochratoxin.177 With the addition of concanavalin A (Con A), glucose oxidase (GO)x-CeO2@Ag-GQDs were also demonstrated to be used as an excellent material by further analyzing the construction of biosensors (Fig. 11a) and the ECL response of different biosensors (Fig. 11b–d) under the same conditions. This ECL system could be used to detect Con A with a high sensitivity.178 Xia and co-workers amplified dual potential ECL signals of GQDs via coupled electrochemical and chemical reactions in the presence of two different reactants (K2S2O8 and Na2SO3); then, CQDs immobilized on graphene showed a nearly 48-fold ECL amplification to detect chlorinated phenols (CPs) in water by using K2S2O8 as a coreactant.179 The superior detection ability results from the high yield of the excited CQDs (C*+) (Fig. 11e).180 The reaction mechanism diagram is shown in Fig. 11f and g. The ECL signal was decreased due to pentachlorophenol (PCP) on the CQDs surface oxidized by C*+. Single-cell analysis is very important in bioscience and biomedical technology. To realize this objective, Yang and co-workers established an ECL analysis platform based on solid-state zinc-coadsorbed CQDs hybrids for breast cancer cell detection.181 Through such a strategy, the determination of MDA-MB-231 and MCF-7 single cells with linear ranges of 1–18 and 1–12 cells has been realized, respectively.
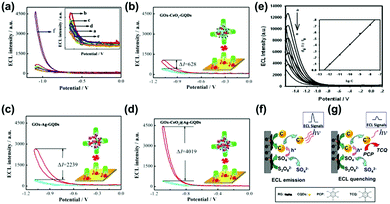 | ||
| Fig. 11 (a) ECL response of different structures of biosensors; a–d in the inset figure stand for bare glassy carbon electrode (GCE), electrodeposit of gold layers (Dp Au)/GCE, GOx/Dp Au/GCE, bovine serum albumin (BSA)/GOx/Dp Au/GCE, Con A/BSA/GOx/Dp Au/GCE and (GOx-CeO2@Ag-GQDs/Con A/BSA/GOx/Dp Au/GCE). (b–d) The ECL responses of different biosensors’ modified electrodes.178 (Reprinted with permission from Elsevier B.V., Copyright 2019.) (e) ECL intensity signal of CQDs/graphene in S2O82− solution (pH 7) at gradient increasing concentrations of pentachlorophenol The mechanism diagram of (f) ECL emission and (g) ECL quenching.180 (Reprinted with permission from the American Chemical Society, Copyright 2013.). | ||
2.7 Chirality
Chirality is defined as a property of a molecule by which it possesses a structure that is non-superimposable on its mirror image, which is one of the most pivotal factors to affect the optical properties of photosensitizer molecules. Chirality is ubiquitous in nature, not only in natural amino acids and DNA, but also in synthesized materials such as CDs.Herranz, Martin and co-workers first reported the preparation of chiral GQDs from acidic exfoliation and oxidation of graphite in the presence of (R) or (S)-2-phenyl-1-propanol.182 Pyrene was introduced to interact with GQDs. The GQDs-(S)/pyrene aggregates represented a positive cotton effect, while the GQDs-E(R)/pyrene aggregates exhibited a negative cotton effect in the circular dichroism spectrum from 320 to 350 nm. This result clearly revealed that the (R) or (S) configuration of 2-phenyl-1-propanol has been introduced into the GQDs structure. Chiral CDs have also been synthesized by using D-/L-methionine, D-/L-glucose, and D-/L-glucosamine.183 The circular dichroism spectrum displayed a peak at around 210 nm in the positive direction for GQDs synthesized from D-methionine, while a different behavior was observed for GQDs from L-methionine. More interestingly, it was found that the chiral GQDs could affect the optical properties of azobenzene. Under UV light, azobenzene can react differently with D- and L-GQDs. Liu and co-workers synthesized chiral emitting CDs through a pyrolytic route using citric acid and D-/L-penicillamine (D/L-Pen) (Fig. 12a).184 The chirality of Pen precursors was transferred to the CDs via an active surface passivation procedure (Fig. 12b). Fig. 12c shows anisotropic D/L-Pen-CDs. Chiral CDs have been widely used for targeted therapy, modulating cellular functions, and photoluminescent enantioselective sensing. Similarly, chiral CDs have also been prepared by cyclic α-amino acids (D- or L-enantiomer), and D-/L-lysine as capping agents.185,186 Recently, the chirality mechanism of D-CQDs and L-CQDs has also been demonstrated (Fig. 12d).187 The spectra of D/L-CQDs and D/L-tryptophan (Trp) were symmetrical in a certain range of wavelengths. Moreover, they were enantiomeric isomers (Fig. 12e and f). The chiral D-/L-CQDs were prepared using raw material D-/L-tryptophan as both carbon and chiral sources. A symmetrical and opposite CDs spectrum has been observed for the L- and D-CQDs, showing three typical bands at 220, 240 and 290 nm. The former 220 nm signal is attributed to the inheritance of L-/D-Trp, and the latter two peaks are related to the inducement of the chiral environment. In detail, the 240 nm signal is ascribed to the π–π* conjugation of the sp2 carbon skeleton in L- and D-CQDs, while the 290 nm signal is derived from the π–π* transition between the introduced indole. A clear understanding of the chiral mechanism provides great potential for their applications in chiral recognition and other fields.
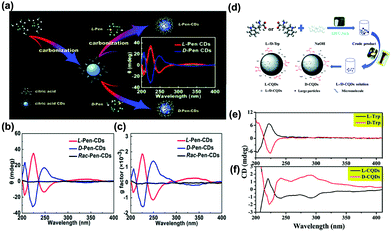 | ||
| Fig. 12 (a) The schematic diagram of the formation of chiral light emitting CDs by citric acid and D/L-Pen as the carbon sources. (b) The circular dichroism spectra of the as-prepared CDs. (c) The corresponding anisotropy factors for all as-prepared CDs.184 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2017.) (d) Schematic for the synthesis of L-/D-CQDs. (e) The circular dichroism spectra of L-/D-Trp. (f) The circular dichroism spectra of L-/D-CQDs.187 (Reprinted with permission from the Royal Society of Chemistry, Copyright 2017.). | ||
3. Summary and outlook
This review provides an overview of the unique optical properties of CDs, including PL, NIR fluorescence, SERS, phosphorescence, CL, ECL, and chirality. As one of the emerging versatile materials, CDs with varied compositions and chemical structures provide tunable and unique optical properties. The distinguishable optical properties have been characterized by the corresponding spectroscopies. Despite realizing the outstanding optical properties of a large number of novel CDs, there are still some challenges to be addressed for guiding their further applications. In particular, more attention should be paid to improve the optical performance of the CDs.Firstly, the synthesis of varied CDs with tunable compositions, sizes, shapes, crystallinity and electronic structures is essential to tailor their optical properties. Although many advances have been achieved to prepare multifunctional CDs, most reported strategies suffer from the shortcomings of time consuming, environmentally unfriendly and low quantity production. Therefore, it is urgent to develop an efficient, controllable and sustainable synthetic strategy to prepare CDs with high quality and a large volume.
Secondly, the PL properties especially with blue or green fluorescence have been broadly explored in most types of CDs. However, CDs with NIR fluorescence, phosphorescence, ECL, and SERS properties are rarely reported. CDs with these optical properties are more suitable for bio-imaging, light emitting and highly sensitive sensing applications. It is highly desirable to prepare unique CDs with such unique optical properties with a high efficiency for promising applications.
Thirdly, the elucidation of the mechanism of the optical properties is still not clear. Up to now, much work has focused on the investigation of the PL mechanism of CDs, which has been attributed to the edge and surface states as well as the quantum confinement effect of CDs. However, there is still no strong evidence to propose a more universally acceptable PL mechanism of the CDs. Most importantly, the mechanisms of the other optical properties of CDs are much less investigated. It is necessary to conduct more theoretical and experimental research to better understand the mechanism of optical properties of CDs.
Finally, the hybridization of CDs with other functional materials is an efficient strategy to enhance their optical properties. In this regard, the modulation of the surface functionalization and interfacial effect of CDs-based hybrids is very important. In addition, much more attention including theoretical calculations and modern characterization techniques should be paid to the investigation of the correlation between the optical properties and their interfacial interactions.
We believe that the deep understanding of the optical properties of CDs will promote their promising applications in a large variety of fields. Furthermore, CDs are considered to be one of the most safe and eco-environmental materials. This makes CDs potential candidates for optical and photoelectronic applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21473068, 21773080) and the Project of Jilin Province Science and Technology Development Plan Project (20180101295JC).Notes and references
- V. K. Voora and K. D. Jordan, Nano Lett., 2014, 14, 4602–4606 CrossRef CAS PubMed.
- G. Brinkmann, J. Goedgebeur and B. D. McKay, J. Chem. Inf. Model., 2012, 52, 2910–2918 CrossRef CAS PubMed.
- H. Li, Q. Zhou, F. Liu, W. Zhang, Z. Tan, H. Zhou, Z. Huang, S. Jiao and Y. Kuang, Appl. Catal., B, 2019, 255, 117755 CrossRef CAS.
- J. Lin, Y. Xu, J. Wang, B. Zhang, C. Wang, S. He and J. Wu, Chem. Eng. J., 2019, 373, 78–85 CrossRef CAS.
- Q. Liu, C. Zeng, Z. Xie, L. Ai, Y. Liu, Q. Zhou, J. Jiang, H. Sun and S. Wang, Appl. Catal., B, 2019, 254, 443–451 CrossRef CAS.
- F. Gorrini, R. Giri, C. E. Avalos, S. Tambalo, S. Mannucci, L. Basso, N. Bazzanella, C. Dorigoni, M. Cazzanelli, P. Marzola, A. Miotello and A. Bifone, ACS Appl. Mater. Interfaces, 2019, 11, 24412–24422 CrossRef CAS PubMed.
- O. Graydon, Nat. Photonics, 2019, 13, 438 Search PubMed.
- F. J. Hsieh, S. Sotoma, H. H. Lin, C. Y. Cheng, T. Y. Yu, C. L. Hsieh, C. H. Lin and H. C. Chang, ACS Appl. Mater. Interfaces, 2019, 11, 19774–19781 CrossRef CAS PubMed.
- M. Švec, P. Merino, Y. J. Dappe, C. González, E. Abad, P. Jelínek and J. A. Martín-Gago, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 121407 CrossRef.
- J. W. Feng, H. M. Ding and Y. Q. Ma, Carbon, 2015, 90, 34–43 CrossRef CAS.
- B. Kang, S. Wu, J. Ma, H. Ai and J. Y. Lee, Nanoscale, 2019, 11, 16599–16605 RSC.
- F. Chang, L. Huang, C. Z. Guo, G. Xie, J. Li and Q. Diao, ACS Appl. Mater. Interfaces, 2019, 11, 35622–35629 CrossRef CAS PubMed.
- L. Bai, H. Yan, Y. Feng, W. Feng and L. Yuan, Chem. Eng. J., 2019, 373, 963–972 CrossRef CAS.
- M. Berenguel-Alonso, I. Ortiz-Gómez, B. Fernández, P. Couceiro, J. Alonso-Chamarro, L. F. Capitán-Vallvey, A. Salinas-Castillo and M. Puyol, Sens. Actuators, B, 2019, 296, 126613 CrossRef CAS.
- X. W. Hua, Y. W. Bao, J. Zeng and F. G. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 32647–32658 CrossRef CAS PubMed.
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- B. B. Chen, M. L. Liu, C. M. Li and C. Z. Huang, Adv. Colloid Interface Sci., 2019, 270, 165–190 CrossRef CAS PubMed.
- M. L. Liu, B. B. Chen, C. M. Li and C. Z. Huang, Green Chem., 2019, 21, 449–471 RSC.
- C. Xia, S. Zhu, T. Feng, M. Yang and B. Yang, Adv. Sci., 2019, 1901316 CrossRef.
- J. Gao, M. Zhu, H. Huang, Y. Liu and Z. Kang, Inorg. Chem. Front., 2019, 4, 1963–1986 RSC.
- C. Hu, M. Li, J. Qiu and Y. Sun, Chem. Soc. Rev., 2019, 48, 2315–2337 RSC.
- J. B. Essner and G. A. Baker, Environ. Sci.: Nano, 2017, 4, 1216–1263 RSC.
- B. B. Chen, Z. X. Liu, W. C. Deng, L. Zhan, M. L. Liu and C. Z. Huang, Green Chem., 2016, 18, 5127–5132 RSC.
- M. L. Liu, L. Yang, R. S. Li, B. B. Chen, H. Liu and C. Z. Huang, Green Chem., 2017, 19, 3611–3617 RSC.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- X. Li, H. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2011, 47, 932–934 RSC.
- S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484–488 RSC.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116–5118 RSC.
- L. Lin and S. Zhang, Chem. Commun., 2012, 48, 10177–10179 RSC.
- W. Wang, C. Damm, J. Walter, T. J. Nacken and W. Peukert, Phys. Chem. Chem. Phys., 2016, 18, 466–475 RSC.
- A. Sciortino, A. Cayuela, M. L. Soriano, F. M. Gelardi, M. Cannas, M. Valcárcel and F. Messina, Phys. Chem. Chem. Phys., 2016, 18, 466–475 RSC.
- S. Tao, T. Feng, C. Zheng, S. Zhu and B. Yang, J. Phys. Chem. Lett., 2019, 10, 5182–5188 CrossRef CAS PubMed.
- F. Wang, S. Pang, L. Wang, Q. Li, M. Kreiter and C. Y. Liu, Chem. Mater., 2010, 22, 4528–4530 CrossRef CAS.
- J. Zong, Y. Zhu, X. Yang, J. Shen and C. Li, Chem. Commun., 2011, 47, 764–766 RSC.
- D. Sun, R. Ban, P. H. Zhang, G. H. Wu, J. R. Zhang and J. J. Zhu, Carbon, 2013, 64, 424–434 CrossRef CAS.
- W. Guan, W. Gu, L. Ye, C. Guo, S. Su, P. Xu and M. Xue, Int. J. Nanomed., 2014, 9, 5071–5078 Search PubMed.
- A. B. Bourlinos, R. Zbořil, J. Petr, A. Bakandritsos, M. Krysmann and E. P. Giannelis, Chem. Mater., 2011, 24, 6–8 CrossRef.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- K. Chen, W. Qing, W. Hu, M. Lu, Y. Wang and X. Liu, Spectrochim. Acta, Part A, 2019, 213, 228–234 CrossRef CAS PubMed.
- K. Lu, C. Li, H. Z. Wang, Y. L. Li, Y. Zhu and Y. Ouyang, J. Photochem. Photobiol., B, 2019, 197, 111504 CrossRef PubMed.
- B. Das, A. Girigoswami, P. Pal and S. Dhara, Mater. Sci. Eng., C, 2019, 102, 427–436 CrossRef CAS PubMed.
- Z. Zhang, X. Wang, X. Zhang, P. Yu, L. Geng and S. Shi, Mater. Res. Bull., 2018, 99, 225–231 CrossRef CAS.
- M. Hashemi, J. Mohammadi, M. Omidi, H. D. C. Smyth, B. Muralidharan, T. E. Milner, A. Yadegari, D. Ahmadvand, M. Shalbaf and L. Tayebi, Mater. Sci. Eng., C, 2019, 103, 109860 CrossRef CAS PubMed.
- Y. Dong, G. Li, N. Zhou, R. Wang, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 8378–8382 CrossRef CAS PubMed.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2011, 133, 15221–15223 CrossRef CAS PubMed.
- V. Tucureanu, A. Matei and A. M. Avram, Crit. Rev. Anal. Chem., 2016, 46, 502–520 CrossRef CAS PubMed.
- T. Susi, T. Pichler and P. Ayala, Beilstein J. Nanotechnol., 2015, 6, 177–192 CrossRef PubMed.
- A. Philippidis, A. Spyros, D. Anglos, A. B. Bourlinos, R. Zbořil and E. P. Giannelis, J. Nanopart. Res., 2013, 15, 1777 CrossRef.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526–9531 RSC.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- C. Lin, Y. Zhuang, W. Li, T. L. Zhou and R. J. Xie, Nanoscale, 2019, 11, 6584–6590 RSC.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572–5575 RSC.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- C. Zhu, S. Yang, J. Sun, P. He, N. Yuan, J. Ding, R. Mo, G. Wang, G. Ding and X. Xie, Synth. Met., 2015, 209, 468–472 CrossRef CAS.
- S. He, M. J. Turnbull, Y. Nie, X. Sun and Z. Ding, Surf. Sci., 2018, 676, 51–55 CrossRef CAS.
- M. P. More, P. H. Lohar, A. G. Patil, P. O. Patil and P. K. Deshmukh, Mater. Chem. Phys., 2018, 220, 11–22 CrossRef CAS.
- J. Ren, F. Weber, F. Weigert, Y. Wang, S. Choudhury, J. Xiao, I. Lauermann, U. Resch-Genger, A. Bande and T. Petit, Nanoscale, 2019, 11, 2056–2064 RSC.
- V. Kumar, V. Singh, S. Umrao, V. Parashar, S. Abraham, A. K. Singh, G. Nath, P. S. Saxena and A. Srivastava, RSC Adv., 2014, 4, 21101 RSC.
- L. Zhang, L. Li, C. Ma, S. Ge, M. Yan and C. Bian, Sens. Actuators, B, 2015, 221, 799–806 CrossRef CAS.
- J. P. Guin, S. K. Guin, T. Debnath and H. N. Ghosh, Carbon, 2016, 109, 517–528 CrossRef CAS.
- H. Xu, S. Zhou, L. Xiao, S. Li, T. Song, Y. Wang and Q. Yuan, Carbon, 2015, 87, 215–225 CrossRef CAS.
- J. Cheng, C.-F. Wang, Y. Zhang, S. Yang and S. Chen, RSC Adv., 2016, 6, 37189–37194 RSC.
- Y. Zhang, J. Zhang, J. Zhang, S. Lin, Y. Huang, R. Yuan, X. Liang and W. Xiang, Dyes Pigm., 2017, 140, 122–130 CrossRef CAS.
- A. Bhati, S. R. Anand, D. Saini, P. Khare, P. Dubey and S. K. Sonkar, New J. Chem., 2018, 42, 19548–19556 RSC.
- F. Huo, W. Liang, Y. Tang, W. Zhang, X. Liu, D. Pei, H. Wang, W. Jia, P. Jia and F. Yang, J. Mater. Sci., 2019, 54, 6815–6825 CrossRef CAS.
- J. Zhao, F. Li, S. Zhang, Y. An and S. Sun, New J. Chem., 2019, 43, 6332–6342 RSC.
- P. Dong, B. P. Jiang, W. Q. Liang, Y. Huang, Z. Shi and X. C. Shen, Inorg. Chem. Front., 2017, 4, 712–718 RSC.
- N. Far'ain Md Noor, M. A. Saiful Badri, M. M. Salleh and A. A. Umar, Opt. Mater., 2018, 83, 306–314 CrossRef.
- K. Zhao, X. Zheng, H. Zhang, M. Xu, S. Wang, Q. Yang and C. Xiong, J. Alloys Compd., 2019, 793, 613–619 CrossRef CAS.
- S. Zhu, Y. Song, J. Wang, H. Wan, Y. Zhang, Y. Ning and B. Yang, Nano Today, 2017, 13, 10–14 CrossRef CAS.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, Microchim. Acta, 2019, 186, 583 CrossRef PubMed.
- L. Ge, N. Pan, J. Jin, P. Wang, G. E. LeCroy, W. Liang, L. Yang, L. R. Teisl, Y. Tang and Y. P. Sun, J. Phys. Chem. C, 2018, 122, 21667–21676 CrossRef CAS.
- M. Yang, H. Mei, Y. Shen, K. Wu, D. Pan, S. Liu, T. Zhang and Y. Zhang, Chem. – Eur. J., 2019, 25, 1–10 CrossRef.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- R. Q. Zhang, E. Bertran and S. T. Lee, Diam. Relat. Mater., 1998, 7, 1663–1668 CrossRef CAS.
- Z. X. Liu, H. Y. Zou, N. Wang, T. Yang, Z. L. Peng, J. Wang, N. Li and C. Z. Huang, Sci. China: Chem., 2018, 61, 490–496 CrossRef CAS.
- M. F. Budyka, Spectrochim. Acta, Part A, 2019, 207, 1–5 CrossRef CAS PubMed.
- T. F. Yeh, W. L. Huang, C. J. Chung, I. T. Chiang, L. C. Chen, H. Y. Chang, W. C. Su, C. Cheng, S. J. Chen and H. Teng, J. Phys. Chem. Lett., 2016, 7, 2087–2092 CrossRef CAS PubMed.
- G. Rajender and P. K. Giri, J. Mater. Chem. C, 2016, 4, 10852–10865 RSC.
- G. Rajender, U. Goswami and P. K. Giri, J. Colloid Interface Sci., 2019, 541, 387–398 CrossRef CAS PubMed.
- J. Yu, C. Liu, K. Yuan, Z. Lu, Y. Cheng, L. Li, X. Zhang, P. Jin, F. Meng and H. Liu, Nanomaterials, 2018, 8, 233 CrossRef PubMed.
- Q. Xu, Q. Zhou, Z. Hua, Q. Xue, C. Zhang, X. Wang, D. Pan and M. Xiao, ACS Nano, 2013, 7, 10654–10661 CrossRef CAS PubMed.
- Q. Fang, Y. Dong, Y. Chen, C.-H. Lu, Y. Chi, H.-H. Yang and T. Yu, Carbon, 2017, 118, 319–326 CrossRef CAS.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260–2345 CrossRef CAS.
- K. K. Chan, C. Yang, Y.-H. Chien, N. Panwar and K.-T. Yong, New J. Chem., 2019, 43, 4734–4744 RSC.
- H. Wang, C. Sun, X. Chen, Y. Zhang, V. L. Colvin, Q. Rice, J. Seo, S. Feng, S. Wang and W. W. Yu, Nanoscale, 2017, 9, 1909–1915 RSC.
- M. A. Sk, A. Ananthanarayanan, L. Huang, K. H. Lim and P. Chen, J. Mater. Chem. C, 2014, 2, 6954–6960 RSC.
- H. Kalita, J. Mohapatra, L. Pradhan, A. Mitra, D. Bahadur and M. Aslam, RSC Adv., 2016, 6, 23518–23524 RSC.
- L. Zhou, J. Geng and B. Liu, Part. Part. Syst. Charact., 2013, 30, 1086–1092 CrossRef CAS.
- Y. H. Yuan, Z. X. Liu, R. S. Li, H. Y. Zou, M. Lin, H. Liu and C. Z. Huang, Nanoscale, 2016, 8, 6770–6776 RSC.
- M. Madhu, T. H. Chen and W. L. Tseng, J. Colloid Interface Sci., 2019, 556, 120–127 CrossRef CAS PubMed.
- S. Yang, J. Sun, X. Li, W. Zhou, Z. Wang, P. He, G. Ding, X. Xie, Z. Kang and M. Jiang, J. Mater. Chem. A, 2014, 2, 8660 RSC.
- X. Sun, H. Liu, L. Yang, X. Wang, W. Yang, M. Wei, X. Liu, J. Cao, J. Yang and S. G. Xing, Nanomaterials, 2018, 8, 635 CrossRef PubMed.
- H. Tetsuka, A. Nagoya, T. Fukusumi and T. Matsui, Adv. Mater., 2016, 28, 4632–4638 CrossRef CAS PubMed.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- J. Zong, X. Yang, A. Trinchi, S. Hardin, I. Cole, Y. Zhu, C. Li, T. Muster and G. Wei, Nanoscale, 2013, 5, 11200–11206 RSC.
- C. Liu, L. Bao, B. Tang, J. Y. Zhao, Z. L. Zhang, L. H. Xiong, J. Hu, L. L. Wu and D. W. Pang, Small, 2016, 12, 4702–4706 CrossRef CAS.
- Q. Xu, R. Su, Y. Chen, S. T. Sreenivasan, N. Li, X. Zheng, J. Zhu, H. Pan, W. Li, C. Xu, Z. Xia and L. Dai, ACS Appl. Nano. Mater., 2018, 1, 1886–1893 CrossRef CAS.
- Y. Xie, J. Zheng, Y. Wang, J. Wang, Y. Yang, X. Liu and Y. Chen, Nanotechnology, 2019, 30, 085406 CrossRef CAS.
- S. Li, Y. Li, J. Cao, J. Zhu, L. Fan and X. Li, Anal. Chem., 2014, 86, 10201–10207 CrossRef CAS PubMed.
- S. Kalytchuk, K. Poláková, Y. Wang, J. P. Froning, K. Cepe, A. L. Rogach and R. Zbořil, ACS Nano, 2017, 11, 1432–1442 CrossRef CAS PubMed.
- Y. Li, G. Bai, S. Zeng and J. Hao, ACS Appl. Mater. Interfaces, 2019, 11, 4737–4744 CrossRef CAS PubMed.
- D. Li, P. Jing, L. Sun, Y. An, X. Shan, X. Lu, D. Zhou, D. Han, D. Shen, Y. Zhai, S. Qu, R. Zboril and A. L. Rogach, Adv. Mater., 2018, 30, e1705913 CrossRef PubMed.
- Y. Zhan, T. Geng, Y. Liu, C. Hu, X. Zhang, B. Lei, J. Zhuang, X. Wu, D. Huang, G. Xiao and B. Zou, ACS Appl. Mater. Interfaces, 2018, 10, 27920–27927 CrossRef CAS.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- Y. Cui, Z. Hu, C. Zhang and X. Liu, J. Mater. Chem. B, 2014, 2, 6947–6953 RSC.
- D. Lu, Y. Tang, J. Gao, Y. Chen and Q. Wang, Dyes Pigm., 2019, 165, 287–293 CrossRef CAS.
- H. D. Ha, M.-H. Jang, F. Liu, Y.-H. Cho and T. S. Seo, Carbon, 2015, 81, 367–375 CrossRef CAS.
- S. Zhuo, M. Shao and S. T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- R. Xie, L. Zhang, H. Xu, Y. Zhong, X. Sui and Z. Mao, Chem. Eng. J., 2017, 310, 79–90 CrossRef CAS.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CrossRef CAS PubMed.
- J. Shen, Y. Zhu, C. Chen, X. Yang and C. Li, Chem. Commun., 2011, 47, 2580–2582 RSC.
- S. Y. Ding, E. M. You, Z. Q. Tian and M. Moskovits, Chem. Soc. Rev., 2017, 46, 4042–4076 RSC.
- M. F. Cardinal, E. Vander Ende, R. A. Hackler, M. O. McAnally, P. C. Stair, G. C. Schatz and R. P. Van Duyne, Chem. Soc. Rev., 2017, 46, 3886–3903 RSC.
- Z. Jie, Y. Zenghe, Z. Xiaolei and Z. Yong, Opt. Express, 2018, 26, 23534–23539 CrossRef CAS PubMed.
- X. Jiang, D. Yin, M. Yang, J. Du, W. Wang, L. Zhang, L. Yang, X. Han and B. Zhao, Appl. Surf. Sci., 2019, 487, 938–944 CrossRef CAS.
- R. Asapu, R. G. Ciocarlan, N. Claes, N. Blommaerts, M. Minjauw, T. Ahmad, J. Dendooven, P. Cool, S. Bals, S. Denys, C. Detavernier, S. Lenaerts and S. W. Verbruggen, ACS Appl. Mater. Interfaces, 2017, 9, 41577–41585 CrossRef CAS PubMed.
- H. Cheng, Y. Zhao, Y. Fan, X. Xie, L. Qu and G. Shi, ACS Nano, 2012, 6, 2237–2244 CrossRef CAS PubMed.
- C. Li, H. Wang, Y. Luo, G. Wen and Z. Jiang, Food Chem., 2019, 289, 531–536 CrossRef CAS PubMed.
- C. Li, P. Fan, A. Liang and Z. Jiang, Mater. Sci. Eng., C, 2019, 99, 1399–1406 CrossRef CAS PubMed.
- X. Feng, C. Li, A. Liang, Y. Luo and Z. Jiang, Nanomaterials, 2019, 9, 480 CrossRef CAS PubMed.
- Y. Fan, H. Cheng, C. Zhou, X. Xie, Y. Liu, L. Dai, J. Zhang and L. Qu, Nanoscale, 2012, 4, 1776–1781 RSC.
- J. Jin, S. Zhu, Y. Song, H. Zhao, Z. Zhang, Y. Guo, J. Li, W. Song, B. Yang and B. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 27956–27965 CrossRef CAS PubMed.
- H. Zhao, Y. Guo, S. Zhu, Y. Song, J. Jin, W. Ji, W. Song, B. Zhao, B. Yang and Y. Ozaki, Appl. Surf. Sci., 2017, 410, 42–50 CrossRef CAS.
- Z. Mao, W. Song, X. Xue, W. Ji, Z. Li, L. Chen, H. Mao, H. Lv, X. Wang, J. R. Lombardi and B. Zhao, J. Phys. Chem. C, 2012, 116, 14701–14710 CrossRef CAS.
- G. Qi, Y. Zhang, S. Xu, C. Li, D. Wang, H. Li and Y. Jin, Anal. Chem., 2018, 90, 13356–13364 CrossRef CAS.
- X. Zhang and X. Du, ACS Appl. Mater. Interfaces, 2016, 8, 1033–1040 CrossRef CAS PubMed.
- P. Y. Cao, Y. L. Liu, Z. T. Lin, J. F. Huang, W. Z. Chen, J. H. Liang, W. Zhou and G. B. Jiang, Carbon, 2017, 118, 625–633 CrossRef CAS.
- D. Liu, X. Chen, Y. Hu, T. Sun, Z. Song, Y. Zheng, Y. Cao, Z. Cai, M. Cao, L. Peng, Y. Huang, L. Du, W. Yang, G. Chen, D. Wei, A. T. S. Wee and D. Wei, Nat. Commun., 2018, 9, 193 CrossRef PubMed.
- G. Baryshnikov, B. Minaev and H. Ågren, Chem. Rev., 2017, 117, 6500–6537 CrossRef CAS PubMed.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751–5753 RSC.
- Q. Li, M. Zhou, Q. Yang, Q. Wu, J. Shi, A. Gong and M. Yang, Chem. Mater., 2016, 28, 8221–8227 CrossRef CAS.
- Q. Li, M. Zhou, M. Yang, Q. Yang, Z. Zhang and J. Shi, Nat. Commun., 2018, 9, 734 CrossRef PubMed.
- J. Tan, Y. Ye, X. Ren, W. Zhao and D. Yue, J. Mater. Chem. C, 2018, 6, 7890–7895 RSC.
- Q. Li, M. Zhou, M. Yang, Q. Yang, Z. Zhang and J. Shi, Nat. Commun., 2018, 9, 734 CrossRef PubMed.
- L. Zhang, R. Zhang, P. Cui, W. Cao and F. Gao, Chem. Commun., 2013, 49, 8102–8104 RSC.
- L. Q. Bai, N. Xue, X. R. Wang, W. Y. Shi and C. Lu, Nanoscale, 2017, 9, 6658–6664 RSC.
- C. Wang, Y. Chen, T. Hu, Y. Chang, G. Ran, M. Wang and Q. Song, Nanoscale, 2019, 11, 11967–11974 RSC.
- W. Li, W. Zhou, Z. Zhou, H. Zhang, X. Zhang, J. Zhuang, Y. Liu, B. Lei and C. Hu, Angew. Chem., Int. Ed., 2019, 58, 7278–7283 CrossRef CAS PubMed.
- K. Jiang, Y. Wang, C. Cai and H. Lin, Adv. Mater., 2018, 30, e1800783 CrossRef PubMed.
- K. Jiang, Y. Wang, X. Gao, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2018, 57, 6216–6220 CrossRef CAS PubMed.
- P. Long, Y. Feng, C. Cao, Y. Li, J. Han, S. Li, C. Peng, Z. Li and W. Feng, Adv. Funct. Mater., 2018, 28, 1800791 CrossRef.
- S. Tao, S. Lu, Y. Geng, S. Zhu, S. A. T. Redfern, Y. Song, T. Feng, W. Xu and B. Yang, Angew. Chem., Int. Ed., 2018, 57, 2393–2398 CrossRef CAS PubMed.
- L. Li, Y. Chen and J. Zhu, Anal. Chem., 2017, 89, 358–371 CrossRef CAS PubMed.
- D. M. Wang, K. L. Lin and C. Huang, Luminescence, 2019, 34, 4–22 CrossRef PubMed.
- J. Zhang, X. Lu, D. Tang, S. Wu, X. Hou, J. Liu and P. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 40808–40814 CrossRef CAS PubMed.
- Z. Lin, W. Xue, H. Chen and J. M. Lin, Anal. Chem., 2011, 83, 8245–8251 CrossRef CAS PubMed.
- R. Sha, A. Gopalakrishnan, K. V. Sreenivasulu, V. V. S. S. Srikanth and S. Badhulika, J. Alloys Compd., 2019, 794, 26–34 CrossRef CAS.
- J. T. Cao, W. S. Zhang, H. Wang, S. H. Ma and Y. M. Liu, Spectrochim. Acta, Part A, 2019, 219, 281–287 CrossRef CAS PubMed.
- J. Wang, M. M. Hassan, W. Ahmad, T. Jiao, Y. Xu, H. Li, Q. Ouyang, Z. Guo and Q. Chen, Sens. Actuators, B, 2019, 285, 302–309 CrossRef CAS.
- S. Rafiei, M. Dadmehr, M. Hosseini, H. A. Kermani and M. R. Ganjali, Methods Appl. Fluoresc., 2019, 7, 025001 CrossRef CAS PubMed.
- S. N. Shah, H. Li and J. M. Lin, Talanta, 2016, 153, 23–30 CrossRef CAS PubMed.
- H. Zhang, X. Zhang and S. Dong, Anal. Chem., 2015, 87, 11167–11170 CrossRef CAS PubMed.
- H. Abdolmohammad-Zadeh and E. Rahimpour, Sens. Actuators, B, 2016, 225, 258–266 CrossRef CAS.
- M. Amjadi, J. L. Manzoori, T. Hallaj and T. Shahbazsaghir, Microchim. Acta, 2017, 184, 1587–1593 CrossRef CAS.
- T. Hallaj, M. Amjadi, Z. Song and R. Bagheri, Mikrochim. Acta, 2017, 185, 67 CrossRef PubMed.
- L. Gao, L. Ju and H. Cui, J. Mater. Chem. C, 2017, 5, 7753–7758 RSC.
- M. Vazquez-Gonzalez, W. C. Liao, R. Cazelles, S. Wang, X. Yu, V. Gutkin and I. Willner, ACS Nano, 2017, 11, 3247–3253 CrossRef CAS PubMed.
- B. Shi, Y. Su, Y. Duan, S. Chen and W. Zuo, Microchim. Acta, 2019, 186, 397 CrossRef PubMed.
- S. Dong, Z. Yuan, L. Zhang, Y. Lin and C. Lu, Anal. Chem., 2017, 89, 12520–12526 CrossRef CAS PubMed.
- D. Li, F. Nie, T. Tang and K. Tian, Microchim. Acta, 2018, 185, 431 CrossRef PubMed.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef CAS PubMed.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- L.-L. Li, J. Ji, R. Fei, C.-Z. Wang, Q. Lu, J.-R. Zhang, L.-P. Jiang and J.-J. Zhu, Adv. Funct. Mater., 2012, 22, 2971–2979 CrossRef CAS.
- H. Xie, F. Bai, Y. Fu, H. Zhu, K. Yan, K. Mao, P. K. Chu, L. Liu and X. Wu, Carbon, 2019, 147, 532–539 CrossRef CAS.
- Z. Guo, T. Hao, S. Du, B. Chen, Z. Wang, X. Li and S. Wang, Biosens. Bioelectron., 2013, 44, 101–107 CrossRef CAS PubMed.
- H. F. Zhao, R. P. Liang, J. W. Wang and J. D. Qiu, Chem. Commun., 2015, 51, 12669–12672 RSC.
- P. Zhang, Z. Xue, D. Luo, W. Yu, Z. Guo and T. Wang, Anal. Chem., 2014, 86, 5620–5623 CrossRef CAS PubMed.
- C. Xiong, W. Liang, H. Wang, Y. Zheng, Y. Zhuo, Y. Chai and R. Yuan, Chem. Commun., 2016, 52, 5589–5592 RSC.
- W. J. Niu, R. H. Zhu, S. Cosnier, X. J. Zhang and D. Shan, Anal. Chem., 2015, 87, 11150–11156 CrossRef CAS PubMed.
- C. Wang, J. Qian, K. Wang, M. Hua, Q. Liu, N. Hao, T. You and X. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 26865–26873 CrossRef CAS PubMed.
- F. Zuo, C. Zhang, H. Zhang, X. Tan, S. Chen and R. Yuan, Electrochim. Acta, 2019, 294, 76–83 CrossRef CAS.
- X. L. Cai, B. Zheng, Y. Zhou, M. R. Younis, F. B. Wang, W. M. Zhang, Y. G. Zhou and X. H. Xia, Chem. Sci., 2018, 9, 6080–6084 RSC.
- S. Yang, J. Liang, S. Luo, C. Liu and Y. Tang, Anal. Chem., 2013, 85, 7720–7725 CrossRef CAS PubMed.
- Y. Qiu, B. Zhou, X. Yang, D. Long, Y. Hao and P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 16848–16856 CrossRef CAS PubMed.
- M. Vazquez-Nakagawa, L. Rodriguez-Perez, M. A. Herranz and N. Martin, Chem. Commun., 2016, 52, 665–668 RSC.
- M. J. Deka and D. Chowdhury, RSC Adv., 2017, 7, 53057–53063 RSC.
- M. Yuan, Y. Guo, J. Wei, J. Li, T. Long and Z. Liu, RSC Adv., 2017, 7, 49931–49936 RSC.
- F. Ostadhossein, G. Vulugundam, S. K. Misra, I. Srivastava and D. Pan, Bioconjugate Chem., 2018, 29, 3913–3922 CrossRef CAS PubMed.
- F. Copur, N. Bekar, E. Zor, S. Alpaydin and H. Bingol, Sens. Actuators, B, 2019, 279, 305–312 CrossRef CAS.
- Y. Wei, L. Chen, J. Wang, X. Liu, Y. Yang and S. Yu, RSC Adv., 2019, 9, 3208–3214 RSC.
| This journal is © the Partner Organisations 2020 |