A sponge-like small pore zeolite with great accessibility to its micropores†
Abstract
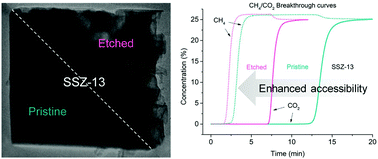
Small pore zeolites, due to pore size constraint, have limited applications. Post-modification on the materials is therefore important to widen their use. A CHA-type zeolite (SSZ-13) with a unique sponge-like structure is obtained by fluoride leaching. The development of macroporosity started from the crystal surfaces and continued progressively into the crystal by prolonging the treatment. Nitrogen physisorption measurements showed an increase in micropore volume and specific surface area as a consequence of the dissolution of the low crystallinity part of the zeolite. The effect of etching on the accessibility through the pore network, evaluated by means of breakthrough experiments on CO2/N2 and CO2/CH4 binary mixtures, showed an improved accessibility thanks to the interconnected macropores which shorten the diffusion pathlength. The set of experimental data shows the sponge-like SSZ-13 crystals retaining the intrinsic zeolitic properties but having an improved accessibility and crystallinity.


 Please wait while we load your content...
Please wait while we load your content...