A systematic study of the optical properties of mononuclear hybrid organo–inorganic lanthanoid complexes†
Abstract
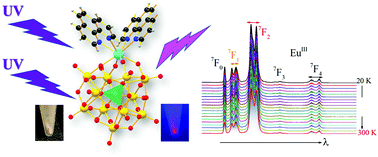
A series of hybrid organo–inorganic mononuclear lanthanoid complexes, [n-NBu4]3[LnH(PW11O39)(phen)2]·H2O, denoted as LM4-1-Ln (Ln = DyIII, TbIII, EuIII, NdIII, ErIII, HoIII and GdIII), were synthesized via hydrothermal synthesis and were structurally characterized by X-ray diffraction. The optical properties of all complexes have been investigated in the solid state. The temperature-dependent emission spectra of LM4-1-Dy, LM4-1-Tb and LM4-1-Eu complexes show intense lanthanoid emissions in the visible region, while LM4-1-Nd shows near-infrared (NIR) luminescence. The EuIII complex shows typical strong red emissions from the 5D0 → 7F0,1,2,3,4 transitions, with the CIE colour coordinates (0.631,0.364), the colour purity value of 83.9% and a quantum yield of up to 4.3%, suggesting that the organic fragment has an effect on the optical properties compared to fully inorganic systems, making this complex very attractive as a red component of light-emitting diodes. The luminescence decays of LM4-1-Dy, LM4-1-Tb and LM4-1-Eu exhibit a biexponential behaviour, with τAV = 4.1(7) μs, 0.35(2) ms and 0.94(3) ms, respectively. The values obtained for Judd–Ofelt intensity parameters Ω2 and Ω4 support the interaction between the EuIII and the ligands. Furthermore, those with ErIII and HoIII present weak emissions in the visible region. The T-dependent photoluminescence results show that the LM4-1-Dy, LM4-1-Tb and LM4-1-Nd complexes have good temperature sensitivity, demonstrating that the materials have the potential to be used as a sensing element for luminescent thermometers in different temperature ranges.


 Please wait while we load your content...
Please wait while we load your content...