Tunable hierarchical surfaces of CuO derived from metal–organic frameworks for non-enzymatic glucose sensing†
Abstract
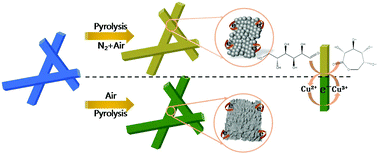
Non-enzyme glucose sensors constructed using transition metal oxides present several advantages such as their low cost, high stability, and high sensitivity. Herein, a kind of porous nanosphere-stacking CuO structure has been synthesized by optimizing the thermal decomposition atmosphere, which was derived from Cu-metal–organic framework (Cu-MOF) microrods. Such hierarchical CuO structures, with controllable porosity and adjustable surface area, are efficient catalytic materials for glucose sensing. Benefiting from structural advantages, the obtained porous hierarchical CuO nanospheres exhibit enhanced sensing performance compared to hierarchical CuO clusters. Based on CuO, the effects of surface and morphology on the sensing performance of glucose are also discussed. The sensitivity of CuO porous hierarchically nanospheres for glucose is found to be 1806.1 μA cm−2 mM−1 in the wide linear range of 0–6.535 mM with a low detection limit (S/N = 3) of 0.15 μM. Glucose detection in artificial saliva is then performed, which shows excellent capability in the low concentration range (5 μM–1.165 mM) for non-invasive sensing performance. The sensor also demonstrates a good recovery in real saliva. The novel MOF-templated CuO hierarchical nanospheres are expected to be effective sensing materials for developing non-enzyme and non-invasive glucose sensors.
- This article is part of the themed collection: 2020 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...