Synthesis of Fe3C@porous carbon nanorods via carbonizing Fe complexes for oxygen reduction reaction and Zn–air battery†
Abstract
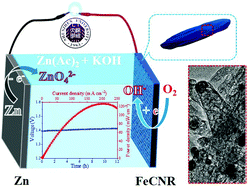
Fe-Based electrocatalysts on carbon substrates are considered suitable candidates for applications in Zn–air batteries due to their favorable ORR performance. Herein, unique Fe3C@N-doped porous carbon nanorods have been synthesized using a soft template method and carbonization. The material proved to have a porous hetero-structure, which is advantageous in providing effective electrocatalysis performance for oxygen reduction reaction, providing broad prospects for the development of non-precious metal electrocatalysts and metal–air batteries. This catalyst used for oxygen reduction reaction exhibits a half-wave of 0.83 V, which is close to that of the commercial Pt/C catalyst. Using the as-prepared Fe3C@N-doped porous carbon nanorods, the open circuit potential and power density of a Zn–air battery has been measured as 1.42 V and 126.4 mW cm−2, respectively, which are better than those of the commercial Pt/C. This study presents a novel strategy to prepare Fe3C@N-doped porous carbon nanorods, which can be used as highly effective oxygen reduction reaction catalysts for Zn–air batteries.
- This article is part of the themed collection: 2020 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...