Joining the journey to near infrared (NIR) imaging: the emerging role of lanthanides in the designing of molecular probes
Guo-Qing
Jin
,
Yingying
Ning
,
Jing-Xing
Geng
,
Zhi-Fan
Jiang
,
Yan
Wang
and
Jun-Long
Zhang
 *
*
Beijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P. R. China. E-mail: zhangjunlong@pku.edu.cn; Fax: +86-10-62767034
First published on 28th October 2019
Abstract
Near-infrared (NIR, 700–1700 nm) bioimaging has gained considerable attention in recent years owing to its high imaging resolution and increasing penetration depth of tissues. NIR emissive lanthanide(III) complexes have emerged as alluring candidates for the design of NIR molecular probes in clinical imaging and diagnosis because of their desirable temporal–spatial resolution, negligible photobleaching effect, long lifetime and low biotoxicity. In this mini-review, we summarize our efforts in the molecular design of NIR luminescent lanthanide porphyrinoids and their biological applications in bioimaging and further clinical diagnosis and treatment. Remarkably, β-fluorinated porphyrin Yb3+ probes exhibit high luminescence (5–13% quantum yields in H2O), long decay lifetimes (>100 μs) and enable in vivo fluorescence intensity imaging and time-resolved fluorescence lifetime imaging (FLIM). Finally, the main concerns and challenges for NIR emissive Ln biolabels such as the diversity of the NIR Ln3+ optical toolbox and their biocompatibility and targeting capability in biological media are discussed so as to facilitate the long-term development of lanthanide chemical biology.
1. Introduction
Near-infrared (NIR, 700–1700 nm) fluorescence imaging has received considerable attention as an emerging tool in clinical diagnosis and therapy owing to its exceptional advantages such as deep tissue imaging with minimum auto-fluorescence interference and high signal-to-background ratios.1–10 Development of NIR probes is essential to extend such an approach to clinical translation and, to date, various NIR probes have been reported including organic fluorophores,1 conjugated copolymers,11 quantum dots (QDs),12 single-walled carbon nanotubes (SWCNTS)13 and rare-earth-doped nanoparticles (RENPs).13,14 In spite of tremendous progress made in the last two decades, the lack of NIR fluorophores with both high quantum yield (QY) and good biocompatibility remains a dominant barrier. For example, small molecular organic fluorophores have considerable advantages such as synthetic flexibility, biocompatibility and biosafety benefited from the small sizes and sophisticated synthetic methodology; however, they suffer from easy photobleaching and low quantum yields.4,5,15 On the other hand, the nanomaterials such as SWCNTS, QDs and RENPS have fascinating photophysical properties, but the potential toxicity or/and metabolism issues, arising from metal ion release and the molecular size effect, hamper their further practical applications.5,7,16 Thus, exploring an alternative approach to construct NIR probes combining the merits of organic small molecules and metal nanoparticles is of importance.To address these issues, we envisaged the utilization of lanthanide (Ln) coordination compounds, which feature metal-centered luminescence arising from the characteristic f–f transitions with good photostability, large Stokes shift, and long lifetime, and provide a toolkit box for NIR probes covering the deep red to NIR region (700–1700 nm).17–20 Since the forbidden nature of Ln3+ f–f transitions results in a low molar absorption coefficient, the crucial choice of the antenna ligand renders the direct excitation of the ligand with a large absorption section capable of achieving intense NIR luminescence. In view of molecular sizes and the related metabolism mechanism, the coordination compounds are more like organic dyes than metal nanoparticles. Moreover, the synthetic flexibility of organic ligands provides access to biocompatible modifications with a well-defined approach.18,21 Thus, NIR emissive Ln complexes are anticipated to stand out as unique optical tags in the NIR toolkit box.
Thanks to the flourishing lanthanide coordination chemistry, various NIR Ln-based molecular probes have been prepared and further applied even in in vivo imaging.22–26 In spite of great progress made in recent years, the development of NIR lanthanide probes is still limited by the low quantum yields (<5%) because the energy transfer efficiency is uncertain and the acceptor excited level of NIR Ln3+ cations is easily quenched by vibrational overtones of X–H (X = C, H, O) oscillators.21,27,28 Our group is interested in mimicking natural tetrapyrrole cofactors, some of which behave like a light harvesting antenna, and thus in developing an antenna ligand to sensitize Ln NIR emission exemplified by ytterbium (Yb3+). Porpholactone shows strong UV-visible absorption and has a unique tetrapyrrolic coordination sphere and tunable triplet state (T1) energy level, and therefore becomes suitable for NIR lanthanide sensitization. In this mini-review, we summarize our recent progress to explore NIR lanthanide complexes for bioimaging and biolabeling. We firstly described the porpholactone chemistry to tailor the unsaturation degree and regioisomerism of the pyrrolic ring to profile the energy transfer efficiency from the triplet state of the ligand to the excited state of Yb3+. Then, we paid attention to remove the high energy vibration of X–H (X = C, N, O) oscillators, affording the NIR luminescent Yb3+ probes with exceptional brightness. Further proof-of-concept applications as optical tags for in vitro and in vivo NIR fluorescence imaging and fluorescence lifetime imaging demonstrated the prospects in NIR bioimaging and diagnosis. Finally, we discuss the remaining synthetic and practical challenges of NIR Ln molecular probes for future studies in lanthanide chemical biology and in vivo applications.29 It is common in luminescent probes research to select “off-the-shelf” fluorophores for whatever application they are targeting, often selecting the moleuclar platforms or nanomaterials that “worked well for someone else”. We hope that with this minireview we can help the researchers appreciate the many considerations in designing biological probes, learn how to evaluate which luminescent form may be suitable for their purposes, and highlight some recent discoveries in NIR Ln research that illustrate some of the key principles for original design of biological molecular probes.
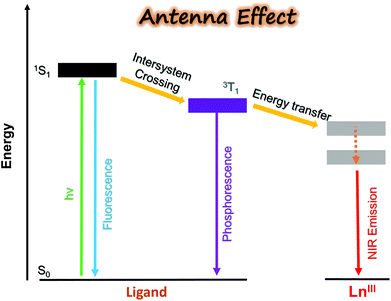
2. Design of the antenna porpholactone ligand: a prerequisite step to sensitize NIR lanthanides
In contrast to visible emissive lanthanide(III) ions such as Eu (red), Tb (green), Sm (orange), and Dy (yellow) applied as biological probes, NIR luminescent Lns (Yb, Nd, Er, etc.) have been much less reported due to their low quantum yields, although they are clinically favorable for their characteristic emission located in the biological transparent window.23 It is difficult to sensitize lanthanides through direct excitation from a high-intensity source, since the Ln3+ f–f electronic transition is forbidden and the molar absorption coefficient is small. Utilization of the “antenna effect”, which means the energy transfer from the ligand triplet state to the upper levels of the lanthanide cation, provides a desirable alternative solution to circumvent the problem (Fig. 1).21 To achieve efficient energy transfer, the energy level of ligand T1 should be higher than the excited state of the lanthanide. Besides, other quenching factors, including back energy transfer (BET), photo-induced electron transfer (PET), and multiphoton relaxation of X–H oscillators (X = C, N, O) should also be considered in the design of NIR lanthanide emitters.So far, various organic ligands have been used for Yb3+ sensitization following the guidance of the Förster/Dexter energy transfer model and/or the energy-gap law.30 Porphyrinoid ligands, featured with large absorption coefficients, good coordination ability, tunable triplet energy states, and convenient chemical modification, have been employed as antenna ligands by many researchers with various auxiliary ligands (Fig. 2a).31
 | ||
| Fig. 2 (a) Typical lanthanide porphyrinate complexes. (b) Porpholactone library constructed by our group. | ||
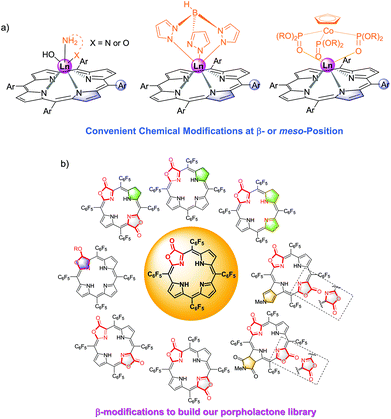
Among them, porpholactone, in which an oxazolone group replaces a pyrrole moiety in a porphyrin macrocycle, has attracted increasing attention since it was firstly reported thirty years ago.32 Our group reported the “RuCl3 + Oxone®” protocol for the concise synthesis of porpholactone on a gram scale, making it much easier to enrich the fascinating porpholactone library for further applications (Fig. 2b).33–35 These types of ligands feature synthetic flexibility by modification of π-porphyrinic periphery, which is effective for tuning the electronic structures including the ground and excited states, and the photophysical properties in the visible to NIR region. In recent years, we summarized our progress in the porpholactone library through tuning the saturation level of π-periphery and the orientation of β-dilactone moieties.36
Preliminary study showed that β-lactonization of the pyrrolic ring could remarkably enhance the Yb3+ NIR emission by 50%–120% and extend its lifetime by narrowing the energy gap between the triplet energy state of porphyrinoids and the emissive state of Yb3+ (Fig. 3a).37 Moreover, we demonstrated the potential applications of water-soluble Yb porpholactone to detect glucose by conjugating glucose to it. Interestingly, the NIR emission of this Yb bioprobe could be specifically switched on by glucose oxidase and then switched off in the presence of glucose, which is the first demonstration that nonpyrrolic porphyrin ligands enhance the QY of Ln luminescence (Fig. 3b).37 This encouraged us to systematically look into the intriguing porpholactone derivatives for NIR Ln sensitization.
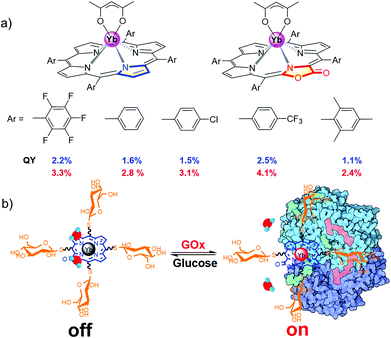 | ||
| Fig. 3 (a) β-Lactonization of porphyrin to enhance the quantum yield of the Yb3+ cation. (b) Chemical structure of the water-soluble Yb porpholactone complex and its sensing for glucose oxidase. Reproduced with permission from ref. 37. Copyright 2014 Wiley-VCH. | ||
3. Design of NIR Yb complexes
3.1. Regioisomeric effect on the sensitization of Yb(III) NIR emission
Naturally occurring chlorophyll b, d, and f with gradually red-shifted Q bands have set us a golden standard to modulate the deep red absorption by altering β-formyl group orientation.38 Inspired by the natural approach, we used porphodilactone as a synthetic model, which enables fine-tuning of the lowest triplet excited states via subtle β-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O configurational isomerism (Fig. 4).39,40 To investigate the bio-inspired orientation effect on NIR Yb sensitization, cis/trans porphodilactones were employed as antenna ligands to coordinate Yb3+ cations.41 Prominently, we found that the Yb3+ NIR emission could be switched “on/off” by the cis/trans porphodilactones with the quantum yields (QYs) of 3.32% and 0.41%, respectively. The difference in QY is mainly due to the energy transfer efficiency from the lowest triplet excited state of the ligand (T1) to the emissive level of Yb3+ (2F5/2,Yb*), as a result of β-substituent orientation alteration.
O configurational isomerism (Fig. 4).39,40 To investigate the bio-inspired orientation effect on NIR Yb sensitization, cis/trans porphodilactones were employed as antenna ligands to coordinate Yb3+ cations.41 Prominently, we found that the Yb3+ NIR emission could be switched “on/off” by the cis/trans porphodilactones with the quantum yields (QYs) of 3.32% and 0.41%, respectively. The difference in QY is mainly due to the energy transfer efficiency from the lowest triplet excited state of the ligand (T1) to the emissive level of Yb3+ (2F5/2,Yb*), as a result of β-substituent orientation alteration.
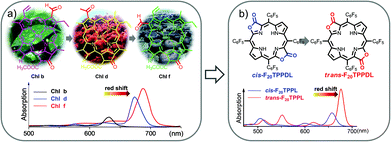 | ||
| Fig. 4 (a and b) The bio-inspired orientation effect on tuning the porphyrinic T1 energy state. Reproduced with permission from ref. 41. Copyright 2014 American Chemical Society. | ||
By estimating the energy level of the T1 state of cis/trans porphodilactones from the phosphorescence spectra of cis-Gd-LOMe and trans-Gd-LOMe at 77 K, we found that the energy gap between the T1 state of cis-porphodilactone and the excited state of Yb* (ca. 1152 cm−1) is much larger than that of the trans-isomer (ca. −25 cm−1). This larger energy gap gives rise to an energy transfer efficiency, according to the “energy gap law”. In addition, to evaluate the effect of a subtle structural change, the spectral overlap between the phosphorescence of cis-Gd-LOMe or trans-Gd-LOMe and the f–f transition absorption of Yb3+ was also investigated. More spectral overlap was found in cis-Yb-LOMe, which is believed to facilitate the energy transfer process and thus NIR emission according to the energy transfer (either Förster or Dexter) mechanism (Fig. 5).
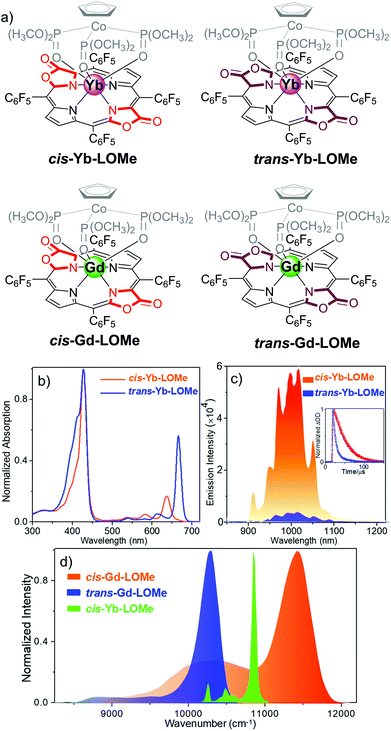 | ||
Fig. 5 (a) Chemical structure of cis-Ln-LOMe and trans-Ln-LOMe (Ln = Yb or Gd). (b) Normalized electronic absorption of cis-Yb-LOMe and trans-Yb-LOMe in DCM. (c) NIR luminescence spectra of cis-Yb-LOMe and trans-Yb-LOMe in DCM at room temperature (inset: decay curve monitored at 970 nm). (d) Normalized phosphorescence emission spectra of cis- and trans-Gd-LOMe at 77 K in CH3OH/C2H5OH (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and f–f transitions 2F7/2 → 2F5/2 of Yb3+ in cis-Yb-LOMe in CD2Cl2. Reproduced with permission from ref. 41. Copyright 2017 American Chemical Society. 1) and f–f transitions 2F7/2 → 2F5/2 of Yb3+ in cis-Yb-LOMe in CD2Cl2. Reproduced with permission from ref. 41. Copyright 2017 American Chemical Society. | ||
Sensitization efficiency is not the only factor to determine the quantum yield of Yb complexes. For example, β-lactonization can promote the energy transfer process between the porphyrinic ligand and the excited state of Yb by narrowing the energy gap (η ∼ 100%); however, the overall quantum yields (QYs) are still low (<5%). According to eqn (1), overall quantum yields are determined by both high sensitization efficiency (η) and large intrinsic quantum yield (ϕLnLn). Thus, the method to achieve a high intrinsic quantum yield is another critical factor.
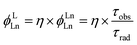 | (1) |
3.2. β-Fluorination: an important approach to achieve a high intrinsic quantum yield
High energy vibration of X–H (X = C, N, O) oscillators from the ligands and solvents are detrimental and can cause quenching of the excited state of Yb3+, which leads to a low intrinsic quantum yield. To remove or reduce detrimental X–H oscillators in the vicinity of the Ln ion, halogenation and deuteration of ligands could be suitable choices.42 A variety of perfluorinated antenna ligands such as bis(perfluorooctylsulfonyl)amide, perdeuterated cryptand, bis(pentafluorophenyl)phosphinic acid and perfluorinated imidodiphosphinate have so far been applied to enhance NIR lanthanide emission especially by extending the lifetimes and intrinsic quantum yields.21 Nevertheless, their ligands have low absorption coefficients and high-lying triplet energy states, which are disadvantageous for the energy transfer process from the ligand to the Ln center. Hence the rational design of a porphyrinoid scaffold by diminishing X–H (X = C, N, O) oscillators is a promising approach to combine the suitable T1 energy levels for the Yb3+ excited state.In 2017, we reported the combination of perfluorinated porphyrin (F28TPP) as the antenna ligand and deuterated Kläui's ligand (NaLOCD3) as the ancillary ligand to significantly improve the QY of NIR Yb(III) emission.43 We could dramatically increase the overall quantum yield of the Yb-F28TPP-LOCD3 complex up to the highest 63% (estimated uncertainty 15%), and extend the lifetime to 714 μs in CD2Cl2 with an intrinsic quantum yield of 75% (Fig. 6). Given the splendid light-harvesting capability (extinction coefficient ε ∼ 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) of the porphyrinoids, this represents the brightest NIR emissive Ln3+ complexes (brightness: ε × ϕ ∼ 190
000 M−1 cm−1) of the porphyrinoids, this represents the brightest NIR emissive Ln3+ complexes (brightness: ε × ϕ ∼ 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) ever reported, making it a fascinating fluorescent lanthanide probe for medical imaging and diagnosis in the NIR biological window.
000 M−1 cm−1) ever reported, making it a fascinating fluorescent lanthanide probe for medical imaging and diagnosis in the NIR biological window.
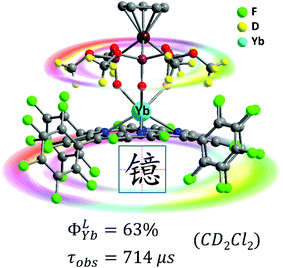 | ||
| Fig. 6 Highly NIR-emissive ytterbium(III) complex Yb-F28TPP-LOCD3 with a quantum yield of up to 63% in CD2Cl2 and a lifetime of up to 714 μs. Reproduced with permission from ref. 43. Copyright 2017 The Royal Society of Chemistry. | ||
In addition to the primary coordination sphere, tuning the secondary coordination sphere is also useful to enhance the NIR lanthanide luminescence but has been much less explored. In 2018, we investigated the sensitization ability of porpholactols with “up/down” isomers, after the reduction of porpholactones by DIBAL.44 For Yb-up, in which the β-hydroxyl group is at the same side of the Yb center, an intramolecular hydrogen bond with the axial Kläui's ligand was easily formed, while Yb-down could not, because the β-hydroxyl group below the porphyrin plane was far away from Kläui's ligand. X-ray diffraction (XRD) analysis suggested that the intramolecular hydrogen bond in Yb-up could shorten the distance between the emissive Yb3+ center and the β-O–H bond, which promoted the adverse non-radiative deactivation of the excited state of the central ion. Thus, Yb-down displayed a much higher quantum yield and longer lifetime (4.5%, 41 μs) than Yb-up (2.4%, 20 μs) due to the weaker vibration quenching effect of the β-O–H bond, demonstrating the role of the secondary coordination sphere on Yb NIR emission (Fig. 7). Interestingly, for high sensitivity of O–H vibration toward temperature and viscosity, Yb-up/down isomers were found to have potential in NIR optical thermometers and viscosity probes. This opens access to the design of NIR lanthanide functional materials sensitive to external or environmental stimuli.
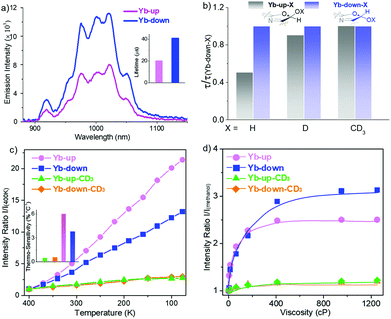 | ||
| Fig. 7 Design of NIR luminescent Yb3+ probes sensitive to environmental stimuli through rationally tuning the secondary coordination sphere. (a) Comparison of NIR emission spectra between Yb-up and Yb-down (λex = 406 nm, A406 nm = 0.1). (b) Decay lifetime of Yb-up-X with reference to Yb down-X (X = H, D, CD3) monitored at 974 nm in CH2Cl2 at room temperature. (c) Emission intensity ratio normalized according to that at 400 K and thermosensitivity (inset) of Yb-up (pink), Yb-down (blue), Yb-up-CD3 (green) and Yb-down-CD3 (orange) in a PMMA thin film (400–77 K) (λex = 406 nm, A406 nm = 0.1). (d) Emission intensity ratio normalized according to that in methanol of Yb-up (pink), Yb-down (blue), Yb-up-CD3 (green) and Yb-down-CD3 (orange) in methanol/glycerol mixtures of different viscosities (λex = 406 nm, A406 nm = 0.1). Reproduced with permission from ref. 44. Copyright 2018 American Chemical Society. | ||
4. NIR lanthanide molecular probes for diagnosis and treatment
4.1. NIR lanthanide optical molecular tags
For NIR emissive lanthanide ions, their unique spectral properties and negligible physical side effects undoubtedly make them promising and competitive labels among the whole clinically useful luminescent toolbox in terms of sensitivity, cost, and user-friendliness.However, at present, NIR Lns such as Yb, Nd and Er have been much less studied compared to visible Lns such as Eu and Tb in bioimaging and bioanalysis. For the development of NIR emissive Ln probes, several groups have contributed pioneering studies. Charbonnière, Seitz and others investigated on improving NIR lanthanide luminescence by extending lanthanides’ radiative lifetime by minimizing the multiphonon relaxation of the harmful X–H bond (X = C, N, O) and by optimizing the energy transfer process by finely tailoring the antenna ligands.45–48 Eliseeva, Petoud and coworkers prepared a polynuclear Sm3+ dendrimer for simultaneous visible and NIR cell imaging. Petoud and coworkers reported the sandwich lanthanide metallocrowns for necrotic cell imaging.24 Wong and co-workers reported a Yb3+ complex conjugated with rhodamine B for NIR-to-visible two photon live cell imaging.25 Borbas and his coworkers reported the first Nd chlorine complex based on a dipicolinic acid (dpa) framework, which can be excited by a Q band.49 Maury and co-workers prepared water-soluble Yb3+ complexes based on acetenyl functionalized triazacyclononane for two photon NIR-to-NIR bioimaging.26,50
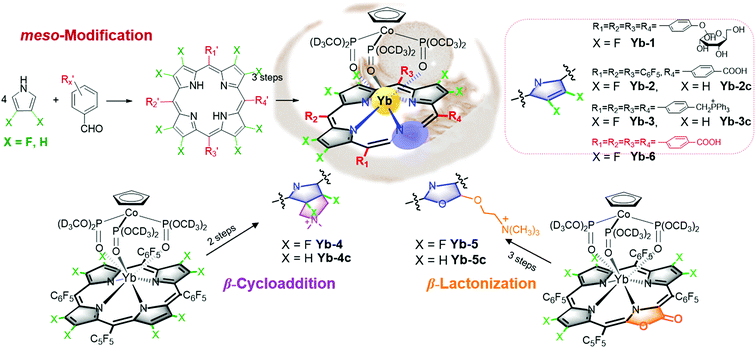
However, all NIR Ln complexes mentioned above suffer from a quite low quantum yield, which calls for our further efforts on developing NIR luminescent Ln probes with both good quantum yield and biocompatibility. The recently reported perfluorinated sandwich porphyrin ytterbium complex Yb-F28TPP-LOCD3 exhibits unprecedented NIR luminescence and stability, but perfluorination makes it too hydrophobic to be taken up by cells.51 Therefore, a biocompatible modification is necessary for the purpose of biological applications (Fig. 8). We firstly embedded the water-soluble functional groups such as glycosyl, carboxyl and triphenylphosphonium, respectively, onto the meso position of porphyrin to obtain water-soluble Yb-1–3. By reduction of the lactone moiety of porpholactones, we attached a hydrophilic quaternary ammonium group to the β-pyrrolic ring. Through β-1,3-dipolar cycloaddition, we could also improve its lipophilicity by attaching the ammonium group. When it comes to cell experiments, we noticed that Yb-5 showed an IC50 value as low as 1.0 μM for HeLa cells, arising from the singlet oxygen generation induced phototoxicity, while Yb-1–4 exhibited negligible cytotoxicity even at 10 μM concentration (cell viability >80%) under light radiation. Interestingly, upon excitation at 420 nm, Yb-2–4 displayed intense intracellular NIR luminescence with preferential colocalization to lysosomes, but Yb-1 was NIR inert, which indicated the effect of biocompatible functionalities on the cellular uptake and intracellular localization.
Notably, we also used water-soluble Yb3+ molecular probes with a quantum yield of ca. 10% in water (Yb-6), which exhibited more than 3 mm penetration depth upon excitation at the Q band. Yb-6 was successfully applied in high resolution whole body, vasculature and lymph node imaging of small mice. This complex metabolized through hepatobiliary and renal systems, like most organic fluorophores. Importantly, we applied Yb-6 in fluorescence-guided sentinel lymph node surgery, which is the first application of NIR-II lanthanide molecular probes in surgical navigation on the mice model (Fig. 9).52
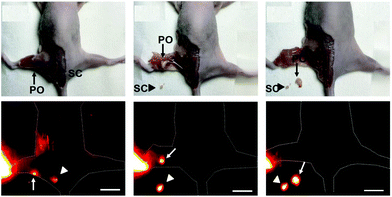 | ||
| Fig. 9 β-Fluorinated Yb3+ complexes were successfully applied in in vivo imaging and they help fluorescence-guided sentinel lymph node surgery. Reproduced with permission from ref. 52. Copyright 2019 The Royal Society of Chemistry. | ||
Compared to the fluorescence intensity imaging, time-resolved fluorescence lifetime imaging (FLIM) does not rely on the local fluorophore concentration and enables quantitative visualization of the physiological environment fluctuation. Ln ions usually have a much longer lifetime (10−6–10−2 s) than biological autofluorescence, thus allowing their application as time-resolved imaging agents. In addition, lanthanide emission is hypersensitive to their coordination sphere, making real-time responsive imaging possible. When it comes to these biocompatible β-fluorinated Yb3+ porphyrinate compounds, they displayed very long decay lifetime on a μs scale, making them ideal τ-probes for FLIM. As shown in the in vitro FLIM experiment of Yb-4/4c (Fig. 10), Yb-4 has a much higher lifetime between 100 and 200 μs than that of Yb-4c (20–40 μs). Interestingly, we found that the lifetime of Yb-4 varies with various subcellular organelles, which can be ascribed to intracellular microenvironment viscosity differences according to the experiments.51 This result greatly inspired us to pursue NIR lifetime τ probes from the β-fluorinated Yb3+ porphyrinic library with high stability, good irreversibility and sensitivity to microenvironment stimuli in vivo.
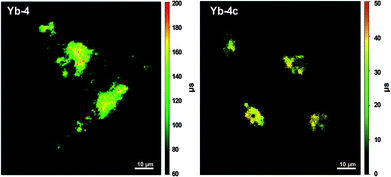 | ||
| Fig. 10 NIR time-resolved image of live HeLa cells incubated with 10 μM Yb-4 and Yb-4c (λex, 405 nm; λem, long pass 850 nm; dwell time, 20 ms). Scale bar: 10 μm. Reproduced with permission from ref. 51. Copyright 2018 The Royal Society of Chemistry. | ||
There are few reports of NIR Ln in vivo FLIM examples so far. In 2015, Thomas and coworkers reported NIR fluorescence lifetime imaging by loading the complexes of Nd3+ and Yb3+ into silicon nanoparticles. In 2017, Maury and coworkers reported a Tb3+ complex via molecular rotation to detect intracellular viscosity but in the visible region.53 We recently reported one upper gastrointestinal pH τ-probe based on a brilliant NIR τ-probe library.54 The upper gastrointestinal pH fluctuation reflects the relevant life metabolism behavior in the human body. Currently, an invasive endoscopy test is a traditional but rather painful method for clinical treatment to monitor gastrointestinal pH values.55 The wide range of pH values in the digestive tract is from 1 to 3 in the stomach to 6–7 in the intestine. In contrast, as a non-invasive method, FLIM can realize the real-time visualization of pH changes in the upper gastrointestinal tract safely, quickly and accurately. Based on the β-fluorinated Yb3+ molecular platform, we developed a NIR fluorescence lifetime τ-probe Yb-6 by attaching four carboxylic groups at the meso position (Fig. 11). With this τ-probe, the pH change is reflected through the difference in the NIR lifetime and intensity.
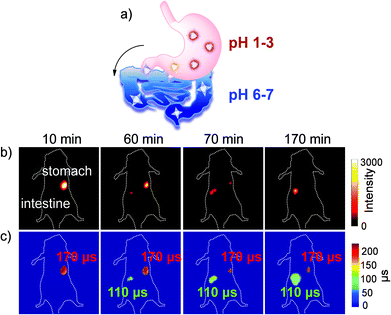 | ||
| Fig. 11 (a) Schematic illustration of the metabolic process of Yb-6 from the stomach to the intestine. (b) NIR fluorescence intensity imaging (exposure time, 25 ms) of the Yb3+ complex. (c) FLIM images (exposure time, 250 ms). λex, 532 nm; λem, 1000 nm longpass. Reproduced with permission from ref. 54. Copyright 2019 The Royal Society of Chemistry. | ||
The Yb-6 was used to report the PH regulation capability of the antiacid drug hydrotalcite chewable tablets in the gastrointestinal tract. Compared with the fuzzy results of fluorescence intensity imaging upon ingestion of hydrotalcite, the fluorescence lifetime of Yb in the stomach showed a reversible change from 170 μs to 130 μs and then back to 170 μs, in accordance with the change of gastric pH from 1.5 to 6.5 to 1.5 (Fig. 12). Therefore, these results indicate that the NIR fluorescent τ-probe can realize the real-time and reversible monitoring of gastrointestinal pH fluctuations, which reveals the prospects of the luminescent β-fluorinated Yb3+ porphyrinates in visualization of in vivo molecular events.
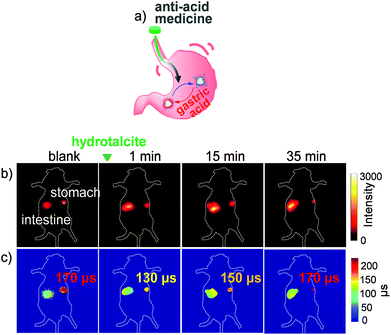 | ||
| Fig. 12 (a) Schematic illustration of monitoring pH changes by Yb-6 in the anti-acid process in the stomach. (b) NIR intensity fluorescence imaging (exposure time, 25 ms). The green triangle at the top indicates the gavage of hydrotalcite. (c) FLIM images (exposure time, 250 ms). λex, 532 nm; λem, 1000 nm long pass. Reproduced with permission from ref. 54. Copyright 2019 The Royal Society of Chemistry. | ||
Lu3+ bears an f14 closed shell electronic structure and large spin–orbit coupling constants (1153 cm−1), which tends to promote the singlet–triplet intersystem crossing (ISC).56 Thus, Lu porphyrinoid complexes are suitable for upconversion based on triplet–triplet annihilation (TTA-UC). Therefore, to enrich the lanthanide-based luminescent toolbox, we also tried to develop porpholactone Lu3+ porphyrinoid photosensitizers for TTA-UC imaging, which is the first attempt to employ lanthanide complexes as photosensitizers for TTA-UC (Fig. 13).57 Generally, TTA processes are bimolecular dynamic quenching processes and rely on diffusional interactions, thus longer triplet lifetimes of either sensitizers or acceptors benefit ΦUC. This hypothesis is supported by the trend of the ΦUC of Lu-1–4, with the lifetime order of Lu-1 > Lu-2 > Lu-3 ≈ Lu-4. Importantly, we could enhance the ΦUC of Lu-1 and Lu-2 in aqueous solutions by encapsulating them to mesoporous silica nanoparticles (MSNs), respectively, which enables live cell imaging and provides a proof-of-concept example of TTA-UC of lanthanide complexes for further biolabelling in in vivo lanthanide chemical biology.
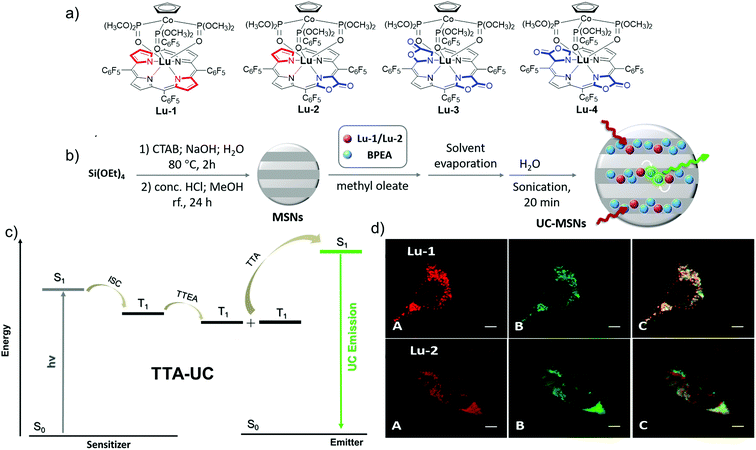 | ||
| Fig. 13 (a) Chemical structures of Lu-1–4. (b) Illustration of the preparation scheme of UC-MSNs. (c) Scheme for TTA-UC via conventional triplet sensitization routes. (d) Confocal fluorescence images of living HeLa cells. Reproduced with permission from ref. 57. Copyright 2018 The Royal Society of Chemistry. | ||
4.2. GdIII porpholactone complexes for the rational design of theranostic agents
Theranostics is an emerging medical treatment, which incorporates both therapeutic and diagnostic agents within the same scaffold and paves a way for personalized medicine design. Based on our previous research, we have reported a series of chlorine-type photosensitizers with different β-ionic terminal groups such as glycosyl, sulfonic and zwitterionic groups and carefully investigated their cellular PDT activities.58 A theranostic agent with photodynamic therapy (PDT) and magnetic resonance imaging (MRI) modalities is really promising for pre-treatment planning, therapy monitoring and treatment outcome assessment, so further research on Ln porphyrinoids is important.59 Based on our porpholactone library, we selected the phototoxic zinc(II) porpholactol as the mother skeleton for PDT functionality, and then introduced β-conjugation of the GdIII DOTA complex for the MRI modality (Fig. 14).60 To our delight, they showed largely enhanced r1 relaxivities compared with that of clinically used MRI contrast agents like GdDOTA and GdDTPA (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate, DTPA = diethylenetriamine pentaacetate), and very good photocytotoxicity towards HeLa cells (IC50 < 2 μM), making them promising candidates for non-invasive theranostic agents with PDT and MRI dual modalities.Importantly, to develop new theranostic agents, it is obviously more attractive to combine diagnostic and therapeutic modalities within a single GdIII molecule entity compared with tedious chemical modifications of commercial MRI contrast agents. The Gd3+ ion has a high-lying f–f transition, so Gd complexes display the population of the T1 state of ligands which are oxygen sensitive.59 In the meantime, the Gd complexes are good candidates for MRI due to their unique magnetic properties arising from the seven unpaired 4f electrons. In 2014, Wong and co-workers reported two Gd complexes with combined PDT and MRI modalities, which can specifically kill HeLa cells via1O2 generation under irradiation but their biocompatibility and 1O2 quantum yield still need to be improved (Fig. 15).61 This remarkable research work inspired our design of next generation dual modal Gd theranostic agents with combined PDT and MRI functionalities. In 2016, we synthesized a series of gadolinium(III) porpholactone complexes and attempted to employ them as dual model theranostic agents with PDT and MRI functionalities (Fig. 15).62 We found that as the porphyrinic ligand triplet state energy level decreased in the order of Gd-1 < Gd-2 < Gd-3 < Gd-4, the gradually narrowed energy gaps could facilitate the energy transfer process from the ligand lowest triplet state to the 1Δg state of 1O2, which significantly facilitates the singlet oxygen (1O2) quantum yield up to 0.99. After glycosidation modifications, Gd-4-Glu became water-soluble and more photocytotoxicity (IC50 = 4.1 μM) was observed than that of the other Gd-1–3-Glu toward HeLa cells in the intracellular PDT experiments, which is highly correlated to the perfect photoinduced 1O2 sensitization capability of Gd-4. For MRI activity, Gd-4-Glu also exhibited increased r1 relaxivity due to the number and orientation differences of the β-lactone moieties in the pyrrolic ring, which also revealed the superiority of our porpholactone framework. Notably, in vivo MRI guided PDT experiments on tumor mice models of Gd-1–4-Glu are also being currently studied in our group.
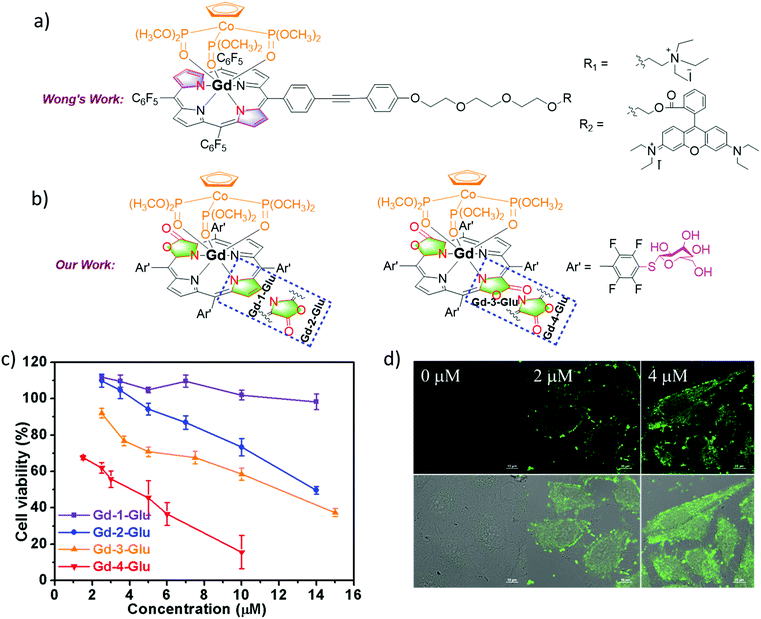 | ||
| Fig. 15 (a) Chemical structure of Wong's work on the design of Gd theranostic agents. (b) Chemical structure of our work on the design of Gd theranostic agents. (c) Photocytotoxicity of Gd-1–4-Glu toward HeLa cells by using a CCK-8 assay under different wavelengths of light irradiation at 600–700 nm with the same dose of light (6.5 mW cm−2) for 30 min, and then cultured in the dark for another 24 h. (d) H2DCFDA fluorescence images of HeLa cells under different drug doses of Gd-2-Glu; 0–2 μM Gd-2-Glu in the dark for 24 h, 10 μM H2DCFDA for 30 min, then irradiated with visible light (400–700 nm, 6.5 mW cm−2) for 30 min. Reproduced with permission from ref. 62. Copyright 2016 Wiley-VCH. | ||
5. Conclusion and outlook
In this mini-review, we summarize the progress in bioinspired porphyrinoid based lanthanide complexes and their applications in bioimaging and biolabelling, and further the attempt to develop theranostic reagents. These results suggest the importance of Ln coordination compounds as NIR imaging molecular probes especially in combination with the merits of organic dyes and metal nanoparticles. In spite of the progress made in the last 5 years, more concerns should be taken into consideration due to the growing importance of lanthanide cations in life science.One is the stability of Ln complexes both in aqueous media and/or upon light irradiation. Biocompatibility is another issue. The current NIR Ln molecular probes are not designed for specific biological targets or events and may not accurately image biological events of interest. To improve their targeting ability, development of bioconjugation with peptides, proteins, and anti-bodies is a desirable strategy. A Ln bioconjugate usually should consist of three parts: the Ln label, which is comprised of a cation and some antenna ligands, an activated functional group that shows high reactivity with targets, and the linker between the Ln complexes and the bioactive moiety. The third challenge is that the excitation wavelength for the NIR lanthanide still needs to be red-shifted to the biological window (700–1000 nm), namely “NIR to NIR”. Despite some pioneering studies revealing the fact that two-photon absorption can facilitate the design of NIR-NIR Ln complexes, the strategy is still limited. Better ligand design is critical to prepare NIR Ln3+ complexes with a broad scope, such as Nd3+, Er3+, Ho3+ and Tm3+, which are rare earth elements that have not been exploited much. Finally, we are also looking forward to more researchers joining the journey to NIR Ln molecular probes and enrich the Ln3+ optical toolbox, thus facilitating further development of lanthanide chemical biology and achieving their medicinal applications in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Scientific Foundation of China (NSFC) (21571007, 21621061, 21778002, and 21861162008) and the National Key Basic Research Support Foundation of China (NKBRSFC) (2015CB856301) for financial support. This work was also supported by the High-performance Computing Platform of Peking University.Notes and references
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 1 CrossRef.
- I. Martinić, S. V. Eliseeva and S. Petoud, J. Lumin., 2017, 189, 19 CrossRef.
- S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258 RSC.
- Kenry, Y. Duan and B. Liu, Adv. Mater., 2018, 30, 1802394 CrossRef.
- C. Li and Q. Wang, ACS Nano, 2018, 12, 9654 CrossRef CAS.
- H. Wan, H. Du, F. Wang and H. Dai, Adv. Funct. Mater., 2019, 1900566, DOI:10.1002/adfm.201900566.
- J. Xu, A. Gulzar, P. Yang, H. Bi, D. Yang, S. Gai, F. He, J. Lin, B. Xing and D. Jin, Coord. Chem. Rev., 2019, 381, 104 CrossRef CAS.
- Y. Fan and F. Zhang, Adv. Opt. Mater., 2019, 7, 1801417 CrossRef.
- H. Wang, X. Mu, J. Yang, Y. Liang, X.-D. Zhang and D. Ming, Coord. Chem. Rev., 2019, 380, 550 CrossRef CAS.
- Q. Miao and K. Pu, Adv. Mater., 2018, 30, 1801778 CrossRef.
- Y. Liu, V. Gunda, X. Zhu, X. Xu, J. Wu, D. Askhatova, O. C. Farokhzad, S. Parangi and J. Shi, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7750 CrossRef CAS.
- I. Medintz, H. T. Uyeda, E. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816 CrossRef CAS.
- H. Dong, S. R. Du, X. Y. Zheng, G. M. Lyu, L. D. Sun, L. D. Li, P. Z. Zhang, C. Zhang and C. H. Yan, Chem. Rev., 2015, 115, 107255 CrossRef.
- F. Ding, Y. Zhan, X. Lu and Y. Sun, Chem. Sci., 2018, 9, 4370–4380 RSC.
- F. Ding, Y. Fan, Y. Sun and F. Zhang, Adv. Healthcare Mater., 2019, 8, e1900260 CrossRef.
- C. P. Montgomery, B. S. Murray, E. J. New, R. Pal and D. Parker, Acc. Chem. Res., 2009, 42, 925 CrossRef CAS.
- S. V. Eliseeva and J. C. Bunzli, Chem. Soc. Rev., 2010, 39, 189 RSC.
- J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729 CrossRef.
- Y. Ning, M. Zhu and J.-L. Zhang, Coord. Chem. Rev., 2019, 399, 213028 CrossRef CAS.
- J.-C. G. Bünzli, Coord. Chem. Rev., 2015, 293–294, 19 CrossRef.
- M. Sy, A. Nonat, N. Hildebrandt and L. J. Charbonnière, Chem. Commun., 2016, 52, 5080 RSC.
- E. Mathieu, A. Sipos, E. Demeyere, D. Phipps, D. Sakaveli and K. E. Borbas, Chem. Commun., 2018, 54, 10021 RSC.
- A. Foucault-Collet, C. M. Shade, I. Nazarenko, S. Petoud and S. V. Eliseeva, Angew. Chem., Int. Ed., 2014, 53, 2927 CrossRef CAS.
- T. Zhang, X. Zhu, C. C. Cheng, W. M. Kwok, H. L. Tam, J. Hao, D. W. Kwong, W. K. Wong and K. L. Wong, J. Am. Chem. Soc., 2011, 133, 20120 CrossRef CAS.
- A. D'Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Angew. Chem., Int. Ed., 2012, 51, 6622 CrossRef.
- C. Anthony and L. J. Zatman, Biochem. J., 1964, 92, 614 CrossRef CAS.
- D. E. Barry, D. F. Caffrey and T. Gunnlaugsson, Chem. Soc. Rev., 2016, 45, 3244 RSC.
- L. J. Daumann, Angew. Chem., Int. Ed., 2019, 58, 12795 CrossRef CAS.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145 CrossRef CAS.
- H. He, Coord. Chem. Rev., 2014, 273, 87 CrossRef.
- M. J. Crossley, J. Chem. Soc., Chem. Commun., 1984, 920 RSC.
- Y. Yu, H. Lv, X. Ke, B. Yang and J.-L. Zhang, Adv. Synth. Catal., 2012, 354, 3509 CrossRef CAS.
- J.-Y. Hu, Z.-Y. Wu, K. Chai, Z.-S. Yang, Y.-S. Meng, Y. Ning, J. Zhang and J.-L. Zhang, Inorg. Chem. Front., 2017, 4, 1539 RSC.
- Y. Yao, Y. Rao, Y. Liu, L. Jiang, J. Xiong, Y. J. Fan, Z. Shen, J. L. Sessler and J. L. Zhang, Phys. Chem. Chem. Phys., 2019, 21, 10152 RSC.
- Y. Ning, G. Q. Jin and J. L. Zhang, Acc. Chem. Res., 2019, 52, 2620 CrossRef CAS.
- X. S. Ke, B. Y. Yang, X. Cheng, S. L. Chan and J. L. Zhang, Chem. – Eur. J., 2014, 20, 4324 CrossRef CAS.
- M. Chen, Annu. Rev. Biochem., 2014, 83, 317 CrossRef CAS.
- X. S. Ke, Y. Chang, J. Z. Chen, J. Tian, J. Mack, X. Cheng, Z. Shen and J. L. Zhang, J. Am. Chem. Soc., 2014, 136, 9598 CrossRef CAS.
- X. S. Ke, H. Zhao, X. Zou, Y. Ning, X. Cheng, H. Su and J. L. Zhang, J. Am. Chem. Soc., 2015, 137, 10745 CrossRef CAS.
- Y. Ning, X. S. Ke, J. Y. Hu, Y. W. Liu, F. Ma, H. L. Sun and J. L. Zhang, Inorg. Chem., 2017, 56, 1897–1905 CrossRef CAS.
- E. Kreidt, C. Kruck and M. Seitz, Handb. Phys. Chem. Rare Earths, 2018, 53, 35 Search PubMed.
- J. Y. Hu, Y. Ning, Y. S. Meng, J. Zhang, Z. Y. Wu, S. Gao and J. L. Zhang, Chem. Sci., 2017, 8, 2702 RSC.
- Y. Ning, Y. W. Liu, Y. S. Meng and J. L. Zhang, Inorg. Chem., 2018, 57, 1332 CrossRef CAS.
- N. Souri, P. Tian, C. Platas-Iglesias, K. L. Wong, A. Nonat and L. J. Charbonnière, J. Am. Chem. Soc., 2017, 139, 1456 CrossRef CAS.
- A. Nonat, S. Bahamyirou, A. Lecointre, F. Przybilla, Y. Mely, C. Platas-Iglesias, F. Camerel, O. Jeannin and L. J. Charbonnière, J. Am. Chem. Soc., 2019, 141, 1568 CrossRef CAS.
- J. W. Caroline Bischof, J. Scholten, S. Trosien and M. Seitz, J. Am. Chem. Soc., 2010, 132, 14334 CrossRef.
- C. Doffek and M. Seitz, Angew. Chem., Int. Ed., 2015, 54, 9719 CrossRef CAS.
- R. Xiong, D. Mara, J. Liu, R. Van Deun and K. E. Borbas, J. Am. Chem. Soc., 2018, 140, 10975–10979 CrossRef CAS.
- A. T. Bui, M. Beyler, A. Grichine, A. Duperray, J. C. Mulatier, Y. Guyot, C. Andraud, R. Tripier, S. Brasselet and O. Maury, Chem. Commun., 2017, 53, 6005 RSC.
- Y. Ning, J. Tang, Y. W. Liu, J. Jing, Y. Sun and J. L. Zhang, Chem. Sci., 2018, 9, 3742 RSC.
- Y. Ning, S. Chen, H. Chen, J.-X. Wang, S. He, Y.-W. Liu, Z. Cheng and J.-L. Zhang, Inorg. Chem. Front., 2019, 6, 1962 RSC.
- A. T. Bui, A. Grichine, A. Duperray, P. Lidon, F. Riobe, C. Andraud and O. Maury, J. Am. Chem. Soc., 2017, 139, 7693 CrossRef CAS.
- Y. Ning, S. Cheng, J. X. Wang, Y. W. Liu, W. Feng, F. Li and J. L. Zhang, Chem. Sci., 2019, 10, 4227 RSC.
- H. Du, M. Liu, X. Yang and G. Zhai, Drug Discovery Today, 2015, 20, 1004 CrossRef CAS.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handb. Photochem., 2006, 353 CAS.
- Z.-S. Yang, Y. Ning, H.-Y. Yin and J.-L. Zhang, Inorg. Chem. Front., 2018, 5, 2291 RSC.
- X.-S. Ke, J. Tang, J.-J. Chen, Z.-Y. Zhou and J.-L. Zhang, ChemPlusChem, 2015, 80, 237 CrossRef CAS.
- S. Jenni and A. Sour, Inorganics, 2019, 7, 10 CrossRef CAS.
- X.-S. Ke, J. Tang, Z.-S. Yang and J.-L. Zhang, J. Porphyrins Phthalocyanines, 2014, 18, 950 CrossRef CAS.
- T. Zhang, R. Lan, C. F. Chan, G. L. Law, W. K. Wong and K. L. Wong, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E5492 CrossRef CAS PubMed.
- X. S. Ke, Y. Ning, J. Tang, J. Y. Hu, H. Y. Yin, G. X. Wang, Z. S. Yang, J. Jie, K. Liu, Z. S. Meng, Z. Zhang, H. Su, C. Shu and J. L. Zhang, Chem. – Eur. J., 2016, 22, 9676 CrossRef CAS.
| This journal is © the Partner Organisations 2020 |