Open Access Article
Open Access ArticleBoosting or moderating surface-initiated Cu(0)-mediated controlled radical polymerization with external additives†
Wei
Li
 a,
Wenbo
Sheng
a,
Rainer
Jordan
*a and
Tao
Zhang
a,
Wenbo
Sheng
a,
Rainer
Jordan
*a and
Tao
Zhang
 *b
*b
aChair of Macromolecular Chemistry, Faculty of Chemistry and Food Chemistry, School of Science, Technische Universität Dresden, Mommsenstr. 4, 01069 Dresden, Germany. E-mail: rainer.jordan@tu-dresden.de
bKey Laboratory of Marine Materials and Related Technologies, Zhejiang Key Laboratory of Marine Materials and Protective Technologies, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, PR China. E-mail: tzhang@nimte.ac.cn
First published on 16th October 2020
Abstract
Surface-initiated Cu(0)-mediated controlled radical polymerization (SI-CuCRP) has been proved to be a powerful method for the fabrication of polymer brushes with various architectures. A key factor in the SI-CuCRP process is the copper disproportionation/comproportionation equilibrium, yet little is known about how the equilibrium works in controlling polymer brush growth, especially under external additives. In this work, the regulation of such equilibrium in SI-CuCRP via external additives is reported in more detail. We find that the polymer brush growth rate can be enhanced via decreasing the ligand concentration or adding NaBr, while it can be reduced by adding ascorbic acid. In addition, the concentration of CuBr2 regulates the ratio of [CuI]/[CuII] to tune the polymer growth kinetics and grafting density over a wide range, which is independent of the distance between the copper plate and initiator-modified substrate. Besides, ultrafast polymerization (surface-initiated hydrogel formation) can be achieved when the ratio of [CuBr2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ligand] reaches 1
[ligand] reaches 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This work gives a deeper insight into the SI-CuCRP process and facilitates the fabrication of polymer brushes on demand.
2. This work gives a deeper insight into the SI-CuCRP process and facilitates the fabrication of polymer brushes on demand.
Introduction
Polymer brushes with tunable physicochemical properties have been intensively used to engineer functional surfaces.1–6 In general, polymer brushes with high grafting density and tailorable functionalities can be fabricated via surface-initiated reversible-deactivation radical polymerization (SI-RDRP).7,8 However, conventional SI-RDRP suffers from poor tolerance to ambient conditions, large volumes of reaction solutions, limitation of substrate size and low end group fidelity which hamper their potential applications.9 To address these problems, various methodologies, such as activation by photochemistry10–13 and electrochemistry,14,15 addition of reducing agents16,17 and enzymes18 and use of copper plates,19 have been employed for polymer brush growth. Among these methods, the use of copper plates (i.e. surface-initiated Cu(0)-mediated controlled radical polymerization, SI-CuCRP) has made significant progress recently since it enables the synthesis of plenty of multifunctional structured polymer brushes with high end group fidelity, over large areas, using a small volume of monomer solution and without a deoxygenation process.20–29 In addition, SI-CuCRP is well compatible with other structuring technologies such as lithography and microfluidics.19,30 Due to these advantages, SI-CuCRP has been used in a variety of applications.31–33 However, the polymerization process and mechanism of SI-CuCRP have not been completely understood yet. Although recent studies have made efforts to uncover the polymerization process and mechanism,22,24,25 few have addressed how the disproportionation/comproportionation equilibrium in SI-CuCRP (Scheme 1a) works in controlling the growth of polymer brushes, especially under external additives. The effects of external additives (e.g. CuBr2, ascorbic acid or NaBr) on the polymerization kinetics of solution RDRP have been well studied in the literature.34 However, the mechanism of SI-CuCRP differs significantly from the solution RDRP since CuI or CuII salts are not added externally and the direct activation of alkyl halides by the Cu plate cannot be realized due to a significant distance of 0.5 mm between the copper plate and the initiator-modified substrate in SI-CuCRP.19,25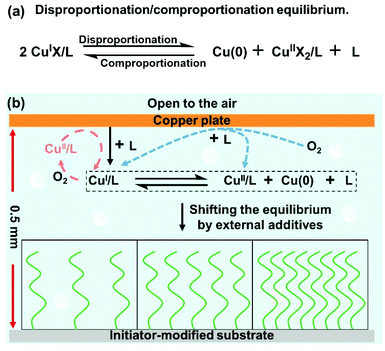 | ||
| Scheme 1 (a) The disproportionation/comproportionation equilibrium. (b) Reaction illustration of SI-CuCRP with variable grafting densities in aqueous media. | ||
In this work, we address the effects of external additives (e.g. CuBr2, ascorbic acid or NaBr) on the polymerization kinetics of SI-CuCRP. We demonstrate that the polymer brush growth rate can be accelerated by the decrease of the ligand concentration (N,N,N′,N′′,N′′-pentamethyldiethylenetriamine, PMDETA) or addition of NaBr and reduced by the addition of ascorbic acid. Furthermore, the polymer growth kinetics and grafting density can be regulated by the variation of CuBr2. It is noteworthy that a surface-grafted hydrogel is formed due to enhanced polymerization when the [CuBr2]/[ligand] ratio reaches 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
Scheme 1b shows the typical reaction process of SI-CuCRP. An initiator-modified substrate was obtained via a self-assembly monolayer according to previous reports.30,35 In normal SI-CuCRP, a copper plate and an initiator-modified substrate of the same size face each other with two 0.5 mm-thick spacers. The system is then immersed into a reaction solution (water, PMDETA as the ligand, and oligo(ethylene glycol)methacrylate (OEGMA) as the monomer, with/without external additives) without deoxygenation. It is worth noting that all the systems are open to air. After a certain time, the substrate covered with POEGMA brushes is thoroughly cleaned with water and the dry thickness of the polymer brushes is measured by ellipsometry. In SI-CuCRP, the copper species (CuI and CuII) are mainly formed via the oxygen-consuming corrosion of metallic copper in the presence of the ligand (Scheme 1b, blue dash).24,25 These CuI species can be oxidized to the CuII species by the dissolved oxygen which can be reduced again to the CuI species by the copper plate via comproportionation (Scheme 1b, red dash).36 Through the two possible ways, SI-CuCRP exhibits high oxygen tolerance. In addition, the CuI species can also form Cu(0) nanoparticles and the CuII species via disproportionation, i.e., there is a disproportionation/comproportionation equilibrium between the copper plate and the substrate, especially in aqueous media.37,38 The copper species (CuI, Cu(0) nanoparticles, and CuII) play important roles in regulating the polymer brush growth in SI-CuCRP. It has been reported that the solvent composition, external CuBr2, the nature of the monomer and ligand concentration dramatically affect the disproportionation/comproportionation equilibrium and the aqueous polymerization.39,40 Therefore, we assumed that the growth of POEGMA brushes could be regulated via shifting the disproportionation/comproportionation equilibrium.
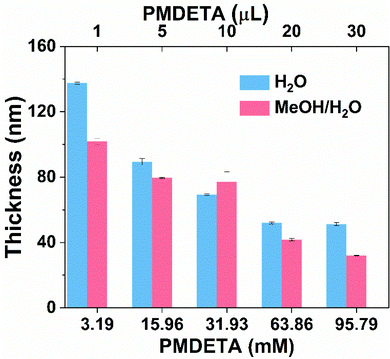
To prove the hypothesis, we first studied the influence of PMDETA concentration on SI-CuCRP in the reaction solution consisting of the ligand, water and monomer (Fig. 1, blue). Under a normal ligand concentration (63.86 mM), the thickness of the polymer brushes was 52 ± 1 nm in 1 h. Further increasing the concentration to 95.79 mM resulted in a slight change in the thickness. When the PMDETA concentration was decreased, it was accompanied by an increase in the thickness of the POEGMA brushes. When the PMDETA concentration decreased to 3.19 mM, thick polymer brushes with a thickness of 138 ± 1 nm were obtained. Further decreasing the concentration to 0.319 mM led to no POEGMA brushes being formed. The reason might be that a very low PMDETA concentration results in very low CuI species, which could be easily depleted by the dissolved oxygen so that no polymer brushes grow. Increasing the PMDETA concentration will enhance the dissolution of the copper plate to form more CuI species, which is enough to deplete the dissolved oxygen and initiate the polymerization. As the concentration of the ligand has little effect on the disproportionation/comproportionation equilibrium in aqueous solution,40 the increase of the CuI species will give more CuII species via disproportionation. As the CuII species are the main deactivators in copper-mediated radical polymerization,34,41 the increase of the CuII species decreases the thickness of the POEGMA brushes, which means that increasing the PMDETA concentration can moderate the SI-CuCRP of OEGMA. In addition, polymerization of OEGMA in a water/methanol mixture was also performed and the result showed the same trend (Fig. 1, red). It is noteworthy that a low PMDETA concentration gave a small grafting area which became larger until the whole substrate was covered with increasing PMDETA concentration (Fig. S1†).
 | ||
| Fig. 1 Influence of the PMDETA concentration on the SI-CuCRP of OEGMA in water or a water/methanol mixture in 1 h without the addition of CuBr2. | ||
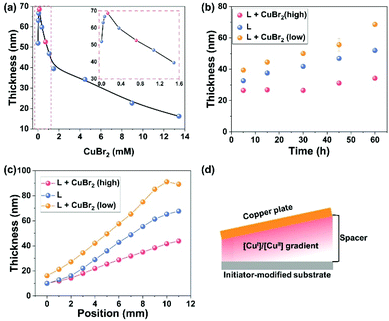
External addition of CuBr2 is another important factor for the SI-CuCRP of OEGMA. With 63.86 mM PMDETA and without the addition of CuBr2, POEGMA brushes can reach 52 ± 1 nm. When CuBr2 was added, the POEGMA brushes exhibited obvious differences which can be divided into three parts by two points (0.13 and 0.72 mM) (Fig. 2a). When 0 < [CuBr2] < 0.13 mM, the addition of CuBr2 speeded up the growth rate. The highest growth rate was at 0.13 mM CuBr2 with 69 ± 1 nm-thick brushes. When the CuBr2 concentration was between 0.13 and 0.72 mM, the thickness of the POEGMA brushes decreased but it was still higher than that under 63.86 mM PMDETA. When the CuBr2 concentration was 0.72 mM, the thickness of the POEGMA brushes was almost the same as that under 63.86 mM PMDETA (52 ± 1 nm). A further increase of the concentration of CuBr2 led to a significant reduction in the growth rate. According to the influence of PMDETA on SI-CuCRP, a high PMDETA concentration will increase the amount of CuI, CuII and Cu(0) species in aqueous media. When a small amount of CuBr2 (0 < [CuBr2] < 0.72 mM) was added, the equilibrium shifted toward comproportionation, and more CuI species were formed leading to the fast growth rate. When adding more CuBr2 ([CuBr2] > 0.72 mM), more CuI species were obtained due to the comproportionation, whereas the introduction of more CuII species as deactivators in the system resulted in a lower growth rate.
We further studied the time dependency of the POEGMA brushes under three different conditions (i.e., 63.86 mM PMDETA (L), 63.86 mM PMDETA + 0.13 mM CuBr2 (L + CuBr2 (low)), and 63.86 mM PMDETA + 4.48 mM CuBr2 (L + CuBr2 (high)), Fig. 2b). No induction period was observed in the three cases. The growth of POEGMA brushes was the fastest under L + CuBr2 (low), followed by L, and L + CuBr2 (high) gave the slowest growth rate. In addition, in the first 30 min, the thickness of the POEGMA brushes under L + CuBr2 (high) exhibited almost no changes, which means that there is no comproportionation during this period in agreement with previous reports.24,42 We also compared the swelling ratios (hswollen/hdry) and grafting densities of the POEGMA brushes obtained under the three conditions (Table 1). The swelling ratios of the POEGMA brushes in 5 min under the three different conditions were about 1.69, 2.54 and 1.89, respectively. As the swelling ratio of the polymer brushes is linearly related to the degree of polymerization, the high swelling ratio is attributed to low grafting density.14,17 According to a previous report,26 the corresponding grafting density under these three conditions is 0.34, 0.18 and 0.28 chains per nm2 with the following order: L > L + CuBr2 (high) > L + CuBr2 (low). As mentioned before, low [CuBr2] yielded more CuI species as activators which would lead to termination, ultimately providing polymer brushes with low grafting density.26,30 The above results also demonstrate that normal SI-CuCRP (in pure PMDETA and without external copper salts) is a well-controlled polymerization method.
| Reaction conditions | h dry (nm) | h swollen (nm) | h swollen/hdry | Grafting density (chains per nm2) |
|---|---|---|---|---|
| a PMDETA 63.86 mM. b PMDETA 63.86 Mm + CuBr2 0.13 mM. c PMDETA 63.86 mM + 4.48 mM CuBr2. d Spin-coated CuBr was used instead of the Cu plate, PMDETA 63.86 mM. Reaction time: 5 min. | ||||
| La | 32.6 ± 0.1 | 55 ± 2 | 1.69 | 0.34 |
| L + CuBr2 (low)b | 39.4 ± 0.6 | 100 ± 3 | 2.54 | 0.18 |
| L + CuBr2 (high)c | 26.4 ± 1 | 50 ± 2 | 1.89 | 0.28 |
| Spin-coated-CuBrd | 11 ± 1 | 14 ± 2 | 1.27 | 0.52 |
Besides the tunable growth rate and grafting densities, gradient polymer brushes were also obtained by tilting the copper plate under these three conditions (Fig. 2c). They showed the same trend of increasing POEGMA thickness with the increasing distance between the copper plate and the initiator-modified substrate as observed before.21,26 Tilting the copper plate produces a [CuI]/[CuII] gradient between the copper plate and the initiator-modified substrate (Fig. 2d). Closer to the copper plate, the concentration of [CuI]/[CuII] was higher, resulting in thinner polymer brushes, which is ascribed to the termination reaction resulting from higher [CuI]/[CuII].25 To further prove the reason, CuBr was spin-coated on a silicon substrate and used as the catalyst to replace the copper plate to perform the polymerization in aqueous media under air. Gradient polymer brushes with the same trend like SI-CuCRP were also obtained (Fig. S2†). High [CuI] resulted in termination and gave thinner polymer brushes. In addition, homopolymer brushes with about 11 ± 1 nm thickness were obtained in 5 min, and no induction period was observed, which further proved the high oxygen tolerance of the sandwiched systems. The swelling ratio of the POEGMA brushes with spin-coated CuBr is about 1.27, which shows a high grafting density with 0.52 chains per nm2 (Table 1). The thicknesses of the gradient POEGMA brushes via spin-coated CuBr range from 0 to 65 nm. In addition, after reaction for 1 h, copper particles were clearly formed on the spin-coated CuBr substrate which was characterized by X-ray diffraction (XRD) (Fig. S3†). The successful fabrication of the POEGMA brushes via spin-coated CuBr shows the high oxygen tolerance of SI-CuCRP, which is mainly attributed to the highly confined sandwiched system. The use of spin-coated CuBr also supports that high [CuI] species leads to termination and polymer brushes with low grafting density.26
Since the initial ratio of [CuBr2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PMDETA] plays an important role in polymerization, we then investigated the influence of ligand concentration on SI-CuCRP in the presence of [CuBr2] = 0.13 mM and the results are summarized in Table 2. When using the initial ratio of [CuBr2]
[PMDETA] plays an important role in polymerization, we then investigated the influence of ligand concentration on SI-CuCRP in the presence of [CuBr2] = 0.13 mM and the results are summarized in Table 2. When using the initial ratio of [CuBr2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PMDETA] = 1
[PMDETA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, surface-initiated hydrogel formation was achieved (Fig. S4†) showing an ultrafast radical polymerization but there were still no free polymers in the reaction solution. The hydrogel is a promising material for surface modification and this method might provide a facile way to fabricate a hydrogel coating via surface-initiated polymerization.43 A further increase of the PMDETA concentration results in homogeneous POEGMA brushes.
2, surface-initiated hydrogel formation was achieved (Fig. S4†) showing an ultrafast radical polymerization but there were still no free polymers in the reaction solution. The hydrogel is a promising material for surface modification and this method might provide a facile way to fabricate a hydrogel coating via surface-initiated polymerization.43 A further increase of the PMDETA concentration results in homogeneous POEGMA brushes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PMDETA] on POEGMA under different conditions
[PMDETA] on POEGMA under different conditions
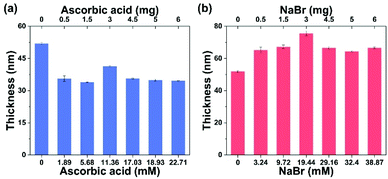
Although the external addition of CuBr2 could greatly modulate SI-CuCRP, using a high concentration of CuBr2 might not be suitable for biological and electronic applications. It is critical to find another way to change the concentration of the CuII species without the introduction of an external copper salt. A widely used reducing agent, AA, commonly used in activators regenerated by electron transfer (ARGET) ATRP,44 was added into the reaction solution (63.86 mM PMDETA, H2O, and OEGMA). The influence of the AA concentration on the growth of the POEGMA brushes is shown in Fig. 3a. When a small amount of AA (1.89 mM) was added into the reaction mixture, the polymerization rate significantly decreased, leading to POEGMA brushes with thicknesses of 35 ± 1 nm. A further increase of the AA concentration did not result in thicker POEGMA brushes. While AA can quickly convert CuII to CuI species, the addition of AA will reduce the CuII species and simultaneously increase the CuI species, both of which will shift the equilibrium toward disproportionation to produce more Cu(0) species. In this case, the increase of the Cu(0) species does not give thicker brushes. Another possible explanation is that the addition of AA might inhibit the desorption of copper species from the copper plate.45 In this way, less copper species were formed from the copper plate and then thinner polymer brushes were obtained. Anyway, AA can be used to moderate SI-CuCRP.
In addition, we also investigated the influence of NaBr on SI-CuCRP (Fig. 3b). Different from AA, the addition of NaBr could promote POEGMA growth. Increasing the [NaBr] showed the highest peak for the fast growth of POEGMA brushes. There might be two reasons for the fast polymer brush growth by the external addition of NaBr: (1) The addition of NaBr could enhance the oxidation of the copper plate to form more CuI and CuII species.45 Accompanied by the disproportionation of the CuI species, a higher concentration of CuII species will be obtained which will lessen the dissociation of the CuII species to result in thicker polymer brushes. (2) The addition of NaBr can directly offset the dissociation of the CuII species in water.46 It is known that the dissociation of the CuII species in water is a very serious challenge, which brings about poor deactivation efficiency. The addition of NaBr improved the deactivation efficiency to achieve well-controlled polymerization leading to thicker polymer brushes.
Based on the above results, a schematic summary of the influence of PMDETA, CuBr2, AA and NaBr on the disproportionation/comproportionation equilibrium and polymer brushes in SI-CuCRP is outlined in Scheme 2. The equilibrium exists in SI-CuCRP, especially in aqueous media. Increasing the PMDETA concentration and adding AA or NaBr shift the equilibrium toward disproportionation while the equilibrium is shifted toward comproportionation by CuBr2. Without or with very low PMDETA, there are no brushes and low PMDETA concentration gives thick polymer brushes while high PMDETA concentration yields thinner brushes (Scheme 2b). For CuBr2, the thickness of the polymer brushes first increases to the highest value and then decreases with the increase in the concentration of CuBr2 (Scheme 2c). The growth of the polymer brushes is moderated by AA (Scheme 2d). The addition of NaBr enhances the growth of the polymer brushes (Scheme 2e).
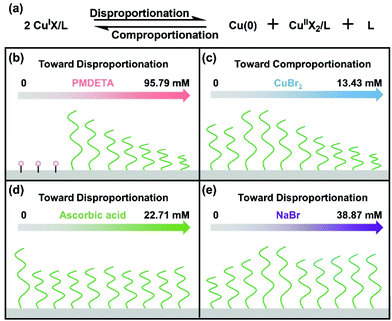 | ||
| Scheme 2 Summary of the regulation of the disproportionation/comproportionation equilibrium (a) and polymer brushes in SI-CuCRP by (b) the ligand, (c) CuBr2, (d) ascorbic acid and (e) NaBr. | ||
Conclusions
In summary, SI-CuCRP of OEGMA in aqueous media under air was systematically investigated via regulating the disproportionation/comproportionation equilibrium in the presence of a ligand and external additives. Decreasing the ligand concentration or adding NaBr could enhance the polymer brush growth, whereas adding ascorbic acid could moderate the polymer brush growth. Moreover, the concentration of CuBr2 also played an important role in tailoring the growth rate and grafting density of the polymer brushes. A surface-grafted hydrogel could be obtained by SI-CuCRP with an optimal [CuBr2]/[PMDETA] ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. These results enhance the controllability of SI-CuCRP in the fabrication of polymer brushes for different fields.
2. These results enhance the controllability of SI-CuCRP in the fabrication of polymer brushes for different fields.
Experimental
Materials
Oligo(ethylene glycol)methyl ether methacrylate (OEGMA, Mn ∼ 500 g mol−1), 2-bromoisobutryl bromide (BIBB), 3-aminopropyltriethoxysilane (APTES), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA), triethylamine (TEA), ascorbic acid (AA), CuBr and NaBr obtained from Sigma-Aldrich were used as received. A PMMA resist 950 K was obtained from Allresist GmbH, Germany. Cu plates (MicroChemicals GmbH, Germany) with a thickness of 200 nm (purity > 99.9%, RMS < 10 nm) were washed with ultrapure water and ethanol under ultrasonication prior to use. After cleaning, the Cu plates were immediately used for reaction. Silicon wafers with an oxide layer (∼300 nm) (Si/SiO2) were purchased from Wacker AG (Burghausen, Germany). Other chemicals were used as received.Preparation of an ATRP-initiator-modified substrate
Si/SiO2 wafers were cleaned for 30 min using an oxygen plasma cleaning system (PDC-002, 200 W from Harrick, USA) with an air flow rate of 50 mL min−1. Then, the clean substrates were immersed in APTES solution (5% in dry acetone). After ultrasonication for 45 min, the substrates were washed with dry acetone and dried with a jet of nitrogen gas. Next, the substrates were immersed in anhydrous dichloromethane (DCM) containing 0.4% TEA and 2% BiBB under argon. The mixture was then stirred at 0 °C for 1 h and at room temperature overnight. Subsequently, the substrates were rinsed with DCM, water, ethanol, and acetone, dried under a stream of nitrogen and then used for polymerization.SI-CuCRP of OEGMA in aqueous media under air
The reaction solution consisted of 0.5 mL of OEGMA, 1.5 mL of water, and different amounts of PMDETA. A copper plate and an ATRP-initiator modified substrate were placed facing each other with two spacers of 0.5 mm. The system was immersed into the reaction solution. After certain reaction time, the substrate was thoroughly cleaned with water and dried with nitrogen to get polymer brushes. Different concentrations of PMDETA were used to study the influence of the ligand on SI-CuCRP. To study other factors (e.g. CuBr2, ascorbic acid, and NaBr), 63.86 mM PMDETA was used.Spin-coated CuBr for polymer brush growth under air
100 mg of CuBr was well dispersed in 1 mL of PMMA solution (PMMA resist 950 K). The obtained mixture was spin-coated on a Si/SiO2 substrate and heated at 90 °C for 5 min and then the spin-coated CuBr substrate was obtained. The process for the polymer brushes using the spin-coated CuBr substrate was almost the same as with SI-CuCRP. Instead of using the copper plate, the spin-coated CuBr substrate was used.Characterization
The thickness of the polymer brushes on the SiO2-wafer was obtained using ellipsometry (SENTECH Instruments GmbH, equipped with a He–Ne laser source with λ = 632.8 nm and a fixed angle of incidence of 60°). The measurements were conducted at least three times. The swelling properties of the POEGMA brushes were measured by atomic force microsopy (AFM) in Milli-Q water. Powder X-ray diffraction (PXRD) was performed at 296(1) K with an X'Pert Pro MPD diffractometer (PANalytical) equipped with a curved Ge(111) monochromator using Cu Kα1 radiation (λ = 154.056 pm).Conflicts of interest
There are no conflicts to declare.Acknowledgements
WL acknowledges the financial support from the China Scholarship Council (CSC) of the People's Republic of China (Ph.D. grant). TZ acknowledges the Ningbo “3315 Innovation Programme” (no. 2019-17-C).Notes and references
- S. Ma, X. Zhang, B. Yu and F. Zhou, NPG Asia Mater., 2019, 11, 24 CrossRef.
- W. Sheng, I. Amin, C. Neumann, R. Dong, T. Zhang, E. Wegener, W. L. Chen, P. Forster, H. Q. Tran, M. Loffler, A. Winter, R. D. Rodriguez, E. Zschech, C. K. Ober, X. Feng, A. Turchanin and R. Jordan, Small, 2019, 15, e1805228 CrossRef.
- K. Matyjaszewski, Adv. Mater., 2018, 30, e1706441 CrossRef.
- W. B. Sheng, W. Li, D. M. Tan, P. P. Zhang, E. Zhang, E. Sheremet, B. V. K. J. Schmidt, X. L. Feng, R. D. Rodriguez, R. Jordan and I. Amin, ACS Appl. Mater. Interfaces, 2020, 12, 9797–9805 CrossRef CAS.
- Q. Wan, M. Liu, J. Tian, F. Deng, G. Zeng, Z. Li, K. Wang, Q. Zhang, X. Zhang and Y. Wei, Polym. Chem., 2015, 6, 1786–1792 RSC.
- W. Sheng, B. Li, X. Wang, B. Dai, B. Yu, X. Jia and F. Zhou, Chem. Sci., 2015, 6, 2068–2073 RSC.
- J. O. Zoppe, N. C. Ataman, P. Mocny, J. Wang, J. Moraes and H.-A. Klok, Chem. Rev., 2017, 117, 1105–1318 CrossRef CAS.
- B. Li, B. Yu, Q. Ye and F. Zhou, Acc. Chem. Res., 2015, 48, 229–237 CrossRef CAS.
- T. Sato, G. J. Dunderdale, C. Urata and A. Hozumi, Macromolecules, 2018, 51, 10065–10073 CrossRef CAS.
- B. Narupai, Z. A. Page, N. J. Treat, A. J. McGrath, C. W. Pester, E. H. Discekici, N. D. Dolinski, G. F. Meyers, J. Read de Alaniz and C. J. Hawker, Angew. Chem., Int. Ed., 2018, 57, 13433–13438 CrossRef CAS.
- J. Yan, B. Li, F. Zhou and W. Liu, ACS Macro Lett., 2013, 2, 592–596 CrossRef CAS.
- J. E. Poelma, B. P. Fors, G. F. Meyers, J. W. Kramer and C. J. Hawker, Angew. Chem., 2013, 125, 6982–6986 CrossRef.
- B. P. Fors, J. E. Poelma, M. S. Menyo, M. J. Robb, D. M. Spokoyny, J. W. Kramer, J. H. Waite and C. J. Hawker, J. Am. Chem. Soc., 2013, 135, 14106–14109 CrossRef CAS.
- B. Li, B. Yu, W. T. Huck, W. Liu and F. Zhou, J. Am. Chem. Soc., 2013, 135, 1708–1710 CrossRef CAS.
- B. Li, B. Yu, W. T. Huck, F. Zhou and W. Liu, Angew. Chem., Int. Ed., 2012, 51, 5092–5095 CrossRef CAS.
- K. Matyjaszewski, H. Dong, W. Jakubowski, J. Pietrasik and A. Kusumo, Langmuir, 2007, 23, 4528–4531 CrossRef CAS.
- J. Yan, B. Li, B. Yu, W. T. Huck, W. Liu and F. Zhou, Angew. Chem., Int. Ed., 2013, 52, 9125–9129 CrossRef CAS.
- L. A. Navarro, A. E. Enciso, K. Matyjaszewski and S. Zauscher, J. Am. Chem. Soc., 2019, 141, 3100–3109 CrossRef CAS.
- T. Zhang, Y. Du, J. Kalbacova, R. Schubel, R. D. Rodriguez, T. Chen, D. R. T. Zahn and R. Jordan, Polym. Chem., 2015, 6, 8176–8183 RSC.
- T. Zhang, E. M. Benetti and R. Jordan, ACS Macro Lett., 2019, 8, 145–153 CrossRef CAS.
- T. Zhang, Y. Du, F. Müller, I. Amin and R. Jordan, Polym. Chem., 2015, 6, 2726–2733 RSC.
- W. Yan, M. Fantin, S. Ramakrishna, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, ACS Appl. Mater. Interfaces, 2019, 11, 27470–27477 CrossRef CAS.
- Y. Du, T. Zhang, D. Gieseler, M. Schneider, D. Hafner, W. Sheng, W. Li, F. Lange, E. Wegener, I. Amin and R. Jordan, Chem. – Eur. J., 2019, 26, 2749–2753 CrossRef.
- W. Yan, M. Fantin, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, ACS Macro Lett., 2019, 8, 865–870 CrossRef CAS.
- M. Fantin, S. N. Ramakrishna, J. Yan, W. Yan, M. Divandari, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, Macromolecules, 2018, 51, 6825–6835 CrossRef CAS.
- E. S. Dehghani, Y. Du, T. Zhang, S. N. Ramakrishna, N. D. Spencer, R. Jordan and E. M. Benetti, Macromolecules, 2017, 50, 2436–2446 CrossRef CAS.
- D. Hafner and R. Jordan, Polym. Chem., 2020, 11, 2129–2136 RSC.
- K. Zhang, W. Yan, R. Simic, E. M. Benetti and N. D. Spencer, ACS Appl. Mater. Interfaces, 2020, 12, 6761–6767 CrossRef CAS.
- Y. Che, T. Zhang, Y. Du, I. Amin, C. Marschelke and R. Jordan, Angew. Chem., Int. Ed., 2018, 57, 16380–16384 CrossRef CAS.
- W. Li, W. Sheng, E. Wegener, Y. Du, B. Li, T. Zhang and R. Jordan, ACS Macro Lett., 2020, 9, 328–333 CrossRef CAS.
- H. Y. Liu, W. L. Chen, C. K. Ober and S. Daniel, Langmuir, 2018, 34, 1061–1072 CrossRef CAS.
- W. L. Chen, M. Menzel, T. Watanabe, O. Prucker, J. Ruhe and C. K. Ober, Langmuir, 2017, 33, 3296–3303 CrossRef CAS.
- R. Nakayama, T. Ube, K. Katayama, M.-a. Haga and T. Ikeda, Mol. Cryst. Liq. Cryst., 2019, 676, 24–29 CrossRef.
- C. Boyer, N. A. Corrigan, K. Jung, D. Nguyen, T. K. Nguyen, N. N. Adnan, S. Oliver, S. Shanmugam and J. Yeow, Chem. Rev., 2016, 116, 1803–1949 CrossRef CAS.
- J. Cui, O. Azzaroni and A. del Campo, Macromol. Rapid Commun., 2011, 32, 1699–1703 CrossRef CAS.
- K. Matyjaszewski, S. Coca, S. G. Gaynor, M. Wei and B. E. Woodworth, Macromolecules, 1998, 31, 5967–5969 CrossRef CAS.
- Q. Zhang, P. Wilson, Z. Li, R. McHale, J. Godfrey, A. Anastasaki, C. Waldron and D. M. Haddleton, J. Am. Chem. Soc., 2013, 135, 7355–7363 CrossRef CAS.
- X. Feng, D. S. Maurya, N. Bensabeh, A. Moreno, T. Oh, Y. Luo, J. N. Lejnieks, M. Galia, Y. Miura, M. J. Monteiro, G. Lligadas and V. Percec, Biomacromolecules, 2020, 21, 250–261 CrossRef CAS.
- F. Alsubaie, A. Anastasaki, V. Nikolaou, A. Simula, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Macromolecules, 2015, 48, 5517–5525 CrossRef CAS.
- F. Alsubaie, A. Anastasaki, V. Nikolaou, A. Simula, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Macromolecules, 2015, 48, 6421–6432 CrossRef CAS.
- D. Konkolewicz, Y. Wang, P. Krys, M. Zhong, A. A. Isse, A. Gennaro and K. Matyjaszewski, Polym. Chem., 2014, 5, 4396–4417 RSC.
- M. E. Levere, N. H. Nguyen and V. Percec, Macromolecules, 2012, 45, 8267–8274 CrossRef CAS.
- S. Ma, C. Yan, M. Cai, J. Yang, X. Wang, F. Zhou and W. Liu, Adv. Mater., 2018, 30, e1803371 CrossRef.
- K. Min, H. Gao and K. Matyjaszewski, Macromolecules, 2007, 40, 1789–1791 CrossRef CAS.
- G. Kılınççeker and S. Çelik, Ionics, 2013, 19, 1655–1662 CrossRef.
- D. Konkolewicz, P. Krys, J. R. Góis, P. V. Mendonça, M. Zhong, Y. Wang, A. Gennaro, A. A. Isse, M. Fantin and K. Matyjaszewski, Macromolecules, 2014, 47, 560–570 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0py01061h |
| This journal is © The Royal Society of Chemistry 2020 |