Synthesis and optoelectronic properties of benzodithiophene-based conjugated polymers with hydrogen bonding nucleobase side chain functionality†
Abstract
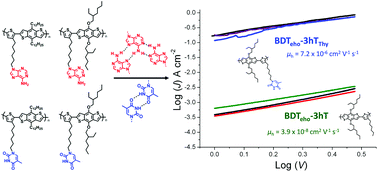
Fundamental properties of conjugated copolymers are sensitively linked to their constitution and features of their repeat unit design that govern assembly and organization across multiple length scales, ultimately impacting device performance. Herein we report the efficient synthesis and characterization, and optical, electrochemical and transport properties of complementary pairs of nucleobase-functionalized, fully conjugated copolymers based on benzo[1,2-b:4,5-b′]-dithiophene (BDT) and 3-hexylthiophene (3hT), which is a quintessential low bandgap polymer. Stille cross-coupling polymerizations enable access to high molecular weight, alternating copolymers of modest dispersity containing pendant adenine or thymine groups in each repeating unit, which provide the capacity to direct molecular assembly. Variations in nucleobase type and design of alkyl side chains on BDT units give rise to a strongly red-shifted absorbance onset for the copolymers containing thymine and adenine functionality in comparison to solution and dried films. Higher hole mobilities were also observed and attributed to the tendency of the nucleobase inclusion to heighten organization. The influence of the nucleobases on the organization is further revealed when thermal pre-treatment is used during film formation: modest heating before casting the non-functionalized BDT-3hT copolymers leads to increases in the mobility, optical absorbance and fluorescence, while copolymers with adenine or thymine pendant groups do not show analogous improvements, suggesting that the nucleobases have promoted nanoscale organization, which is consistent with assessments of hydrogen bonding interactions. Given the structure-directing ability of nucleobases through hydrogen bonding, π-stacking, and complementary base pairing, these novel materials engender myriad opportunities to examine how specific molecular-level interactions that cue self-assembly affect optoelectronic properties and device-level performance.


 Please wait while we load your content...
Please wait while we load your content...