Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct laser writing of poly(phenylene vinylene) on poly(barrelene)†
Tang
Tang
a,
Guillermo
Ahumada
 a and
Christopher W.
Bielawski
a and
Christopher W.
Bielawski
 *ab
*ab
aCenter for Multidimensional Carbon Materials (CMCM), Institute for Basic Science (IBS), Ulsan 44919, Republic of Korea. E-mail: bielawski@unist.ac.kr; Tel: +82-52-217-2952
bDepartment of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea
First published on 21st July 2020
Abstract
The ring-opening metathesis polymerization of barrelene (bicyclo[2.2.2]octa-2,5,7-triene) is described. The monomer was synthesized using an optimized route and characterized, for the first time, using X-ray diffraction analysis of a coordination complex. The solubility of poly(barrelene) was found to correlate with the ratio of cis to trans exocyclic vinylenes in the polymer backbone. Copolymers of barrelene and norbornene were also prepared and used to obtain robust films. The barrelene-containing polymers underwent spontaneous dehydrogenation under air or upon being subjected to laser pulses to afford poly(phenylene vinylene). A series of well-defined patterns with micrometer dimensions were created by direct laser writing on films of poly(barrelene-co-norbornene) and visualized by the fluorescence of the conjugated polymer that formed upon irradiation.
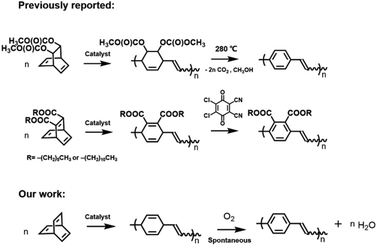
Conjugated polymers1–3 have been used in a wide range of electronic4,5 and optical applications6–8 due to their abilities to form films and exhibit high electrical conductivities upon oxidative or reductive doping.9 Although poly(acetylene)10–13 is the prototypical example, significant attention has been directed toward poly(phenylene vinylene) (PPV) because of its low optical band gap, large nonlinear optical response, and emissive characteristics. While these properties poise PPV for use in applications that range from light-emitting diodes to photovoltaic devices,6,14,15 the material is typically intractable and therefore challenging to process which mandates the need for functionalized polymeric precursors in conjunction with a post-polymerization modification scheme to enhance solubility.16 The precursors are typically synthesized using step-growth16–21 or chain-growth polymerization methodologies,22,23 and then heated or chemically-treated to promote transformation. For example, as summarized in Scheme 1, Grubbs reported that the ring-opening metathesis polymerization (ROMP) of a bis(carboxylate) functionalized bicyclo[2.2.2]octadiene followed by a thermally-induced elimination at an elevated temperature (280 °C) affords PPV.24 Substituted PPVs have been obtained in similar manner by treating polymers obtained via ROMP25,26 with relatively harsh oxidants (e.g., 2,3-dichloro-5,6-dicyanoquinone; DDQ).
A new approach to PPV was envisioned through the ROMP of “barrelene” (bicyclo[2.2.2]octa-2,5,7-triene) (1). The corresponding polymer, which is formally a copolymer that consists of alternating 1,4-cyclohexadienyl and vinylene repeat units, should be prone to aromatization via dehydrogenation and thus may transform to PPV under relatively mild conditions. The realization of such a feature combined with the emissive characteristic of PPV was expected to enable the use of poly(barrelene) in laser machining, direct laser writing (DLW) and other contemporary patterning technologies that create micro- or nanometer sized objects in a manner that does not require masks, inks or developing agents.27–29 While metallic nanoclusters are often utilized as substrates in such applications, metal-free materials are appealing alternatives from economic and fundamental perspectives. Substrates that afford conjugated polymers, particularly those that luminesce, are especially attractive because they should facilitate access to novel types of high precision optical devices, including displays, sensors, and waveguides. For example, Mendonça described the synthesis of spatially resolved PPV by applying laser pulses to films of poly(xylyliden tetrahydrothiophenium chloride).30 Photon absorption was shown to result in a photo-thermal reaction that facilitated the elimination of reactive byproducts, including sulfonium leaving groups and hydrochloric acid, and afforded PPV with a higher conjugation length than analogues prepared using conventional thermal-based approaches.
Herein the ROMP of barrelene is described. The solubility of the resulting polymer was found to depend on its geometry with the cis form exhibiting a higher solubility in organic solvents than its trans isomer. Copolymers of barrelene and norbornene were also synthesized and used to create robust films. Exposure of the polymers to air was found to result in spontaneous dehydrogenation and afforded PPV. The transformation was also facilitated using laser pulses and DLW on films of poly(barrelene-co-norbornene) afforded precisely defined fluorescent patterns with micrometer-sized dimensions.
The requisite monomer (1) has a storied history. It was first called “klovosene” (after a Greek word for bird-cage) by Hine in 1955.31 However, Zimmerman coined the term “barrelene” after synthesizing the molecule in 1960 because its molecular orbitals resembled the shape of a wooden barrel.32 The π-system features Möbius topology and was initially hypothesized to result in a new type of aromaticity.33 However, transannular interactions lead to overlap of sets of p-orbitals with mismatched signs33 and thus minimizes any stabilization from such an arrangement.33 Indeed, barrelene reacts in a manner typical of alkenes: it readily undergoes additions with bromine or hydrogen33 and is prone to epoxidation.34
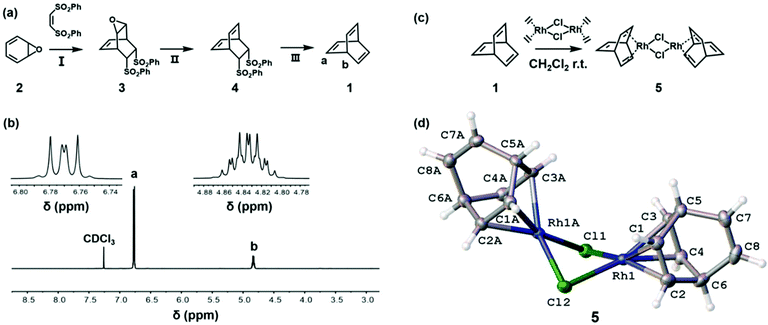
As summarized in Fig. 1a, barrelene (1) was synthesized according to modifications of literature reports.35,36 Heating a mixture of benzene oxide (2)36 and cis-1,2-bis(phenylsulfonyl)ethylene at 80 °C in toluene afforded the corresponding cycloadduct after 24 h. Subsequent reduction with WCl6 and n-butyllithium (n-BuLi) in THF afforded 4, which was desulfurized with sodium amalgam to afford 1.35 Spectroscopic data recorded for the product agreed with literature reports.33,35 A representative 1H NMR spectrum is shown in Fig. 1b.
Although barrelene has been known for decades, the structure of the compound has been determined using only spectroscopic techniques. It was reasoned that a solid-state structure may be obtained through the formation of a coordination complex. Analogous to methodology used to form [Rh(norbornadiene)Cl]2,37,38 mixing excess barrelene and di-μ-chlorotetraethylene dirhodium(I) in CH2Cl2 afforded [Rh(barrelene)Cl]2 (5) (Fig. 1c). Crystals were obtained by slowly evaporating a mixture of CH2Cl2 and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) that was saturated with 5. As shown in Fig. 1d, X-ray diffraction analysis of the crystals revealed that each Rh(I) atom was chelated by a molecule of barrelene and interconnected via chloride bridges. The average distance between the carbon atoms in the alkenes coordinated to the Rh atoms was measured to be 1.3965 ± 0.010 Å, consistent with the value reported for [Rh(norbornadiene)Cl]2 (1.396 ± 0.003 Å).38 The average bond lengths of the non-coordinated alkenes were measured to be relatively short (1.316 ± 0.001 Å) due to the lack of π-backbonding interactions. To the best of our knowledge, this is first example of an organometallic complex that contains barrelene and provides unambiguous solid-state structural elucidation of the compound.
1 v/v) that was saturated with 5. As shown in Fig. 1d, X-ray diffraction analysis of the crystals revealed that each Rh(I) atom was chelated by a molecule of barrelene and interconnected via chloride bridges. The average distance between the carbon atoms in the alkenes coordinated to the Rh atoms was measured to be 1.3965 ± 0.010 Å, consistent with the value reported for [Rh(norbornadiene)Cl]2 (1.396 ± 0.003 Å).38 The average bond lengths of the non-coordinated alkenes were measured to be relatively short (1.316 ± 0.001 Å) due to the lack of π-backbonding interactions. To the best of our knowledge, this is first example of an organometallic complex that contains barrelene and provides unambiguous solid-state structural elucidation of the compound.
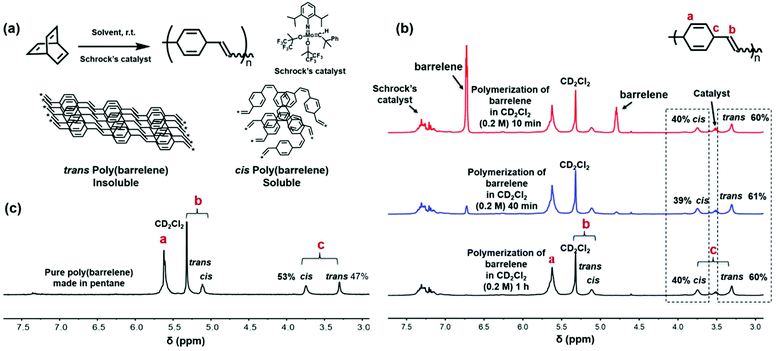
As summarized in Fig. 2a, barrelene was polymerized using Schrock's catalyst, 2,6-diisopropylphenylimidoneophylidene molybdenum(VI) bis(hexafluoro-t-butoxide).39 Treatment of a solution of 1 in CH2Cl2 ([1]0 = 1.0 M) with the catalyst ([1]0/[cat.]0 = 50) at ambient temperature and under an atmosphere of nitrogen afforded an insoluble product after 5 min. Similar results were obtained when the initial monomer concentration was decreased (0.2 M) and when different solvents (e.g., CH2Cl2, CHCl3, or THF) were used. To gain additional insight, a polymerization reaction ([1]0/[cat.]0 = 50, [1]0 = 0.2 M, CD2Cl2) was monitored using 1H NMR spectroscopy. As shown in Fig. 2b, barrelene appeared to convert to a polymer consistent with the expected structure over time; however, the ratio of the signals assigned to the catalyst and the signals assigned to the soluble product remained constant after normalization (4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), even as the reaction proceeded which indicated that the polymer began to precipitate once a certain molecular weight threshold was reached. Inspection of the NMR data also revealed that the exocyclic vinylene content in the polymer that formed was approximately 60% trans. Thus, it was reasoned that the insoluble products may due to the relatively high trans alkene content, which facilitates stacking of the polymer chains and reduces solubility.
1.0), even as the reaction proceeded which indicated that the polymer began to precipitate once a certain molecular weight threshold was reached. Inspection of the NMR data also revealed that the exocyclic vinylene content in the polymer that formed was approximately 60% trans. Thus, it was reasoned that the insoluble products may due to the relatively high trans alkene content, which facilitates stacking of the polymer chains and reduces solubility.
Since greater than 50% of the exocyclic vinylenes in the polymer backbone were observed to be trans, it was surmised that the catalyst facilitates the conversion of the cis forms of the units to their thermodynamically more stable trans isomers. To potentially form a polymer with a relatively high cis alkene content, the polymerization reaction was conducted in pentane as it was envisioned that this would be a poor solvent for the polymer and thus would facilitate precipitation prior to isomerization. Performing the ROMP in pentane using the aforementioned conditions ([1]0/[cat.]0 = 50, [1]0 = 0.2 M) resulted in the rapid formation of a precipitate, which was subsequently collected in a 75% yield based on the structure of poly(1). The polymer was found to be soluble in organic solvents (e.g., THF, CH2Cl2 and CHCl3) and featured a relatively high exocyclic cis vinylene content (53%) as determined by 1H NMR spectroscopy in CD2Cl2 (Fig. 2c). The number average molecular weight (Mn) and polydispersity index (Đ) of the polymer were measured to be 2400 Da and 2.0, respectively, by size exclusion chromatography (SEC) (Fig. S19†).
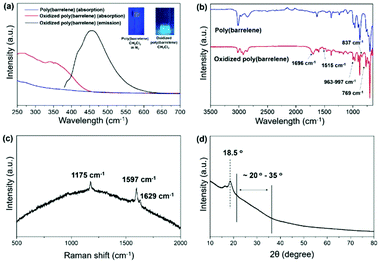
Exposure of poly(barrelene) to air had marked effects on its chemical and physical properties. For example, the solubility of the material appeared to gradually decrease upon exposure to air and was accompanied by color changes to yellow and then brown. In addition, while freshly prepared polymer was non-emissive, a green emission was observed when aerated CH2Cl2 solutions of the material were excited with UV light (λex = 365 nm) over several days. The observations prompted us to hypothesize that poly(barrelene) was undergoing spontaneous oxidation to PPV. To test the hypothesis, UV-vis spectra were recorded for freshly prepared poly(barrelene) and after the material was exposed to aerated CH2Cl2 for 1 month. As shown in Fig. 3a, the UV/vis spectrum recorded for the latter exhibited an absorbance profile with a local maximum (λmax) of 339 nm. The product was also found to fluoresce with a maximum at 463 nm (λex = 365 nm) as determined by spectrofluorimetry. For comparison, PPVs have been reported to absorb and emit over the ranges of 400–442 nm and 489–588 nm, respectively, under similar conditions.26 Other spectroscopic and crystallography data were also consistent with the formation of PPV. As shown in Fig. 3b, FT-IR analysis revealed that the oxidized product displayed signals at 1696 cm−1, 1515 cm−1 and 837 cm−1 which were diagnostic of aryl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, and C–H stretching modes.40,41 Signals were also observed at 962–997 cm−1 and 769 cm−1, and in accord with the trans- and cis-vinylene C–H out-of-plane bending modes, respectively, of PPV. Likewise, as shown in Fig. 3c, the salient Raman signals recorded at 1175 cm−1, 1597 cm−1 and 1629 cm−1 matched literature reports for PPV.30,42 A powder X-ray diffraction analysis revealed that the oxidized product was highly crystalline,43 as indicated by the strong signal observed at 18.5°, and the broad signal recorded between 20° and 35° was agreement with literature data for PPV (Fig. 3d).41
C, C–C, and C–H stretching modes.40,41 Signals were also observed at 962–997 cm−1 and 769 cm−1, and in accord with the trans- and cis-vinylene C–H out-of-plane bending modes, respectively, of PPV. Likewise, as shown in Fig. 3c, the salient Raman signals recorded at 1175 cm−1, 1597 cm−1 and 1629 cm−1 matched literature reports for PPV.30,42 A powder X-ray diffraction analysis revealed that the oxidized product was highly crystalline,43 as indicated by the strong signal observed at 18.5°, and the broad signal recorded between 20° and 35° was agreement with literature data for PPV (Fig. 3d).41
To increase the stability of the barrelene-containing polymers and to facilitate the use of barrelene in optical applications (see below), a series of copolymers of 1 and a relatively inert monomer (norbornene) were synthesized. Solutions of norbornene (6) and 1 ([6]0/[1]0 = 6 or 15, [6 + 1]0 = 0.4 M) in CH2Cl2 were independently treated with catalyst ([6 + 1]0/[cat.]0 = 300) for 2 h at ambient temperature under an atmosphere of N2 gas. The polymerization reactions were subsequently quenched after 2 h with methanol, and then the resulting product mixtures were poured into cold methanol. The precipitated solids were collected, dissolved in CH2Cl2, and reprecipitated from pentane to obtain products that were isolated in high yield (89–91%) based on the structures of the corresponding copolymers (7) (Fig. 4a). Analysis of 7 by 1H NMR spectroscopy revealed that the ratio of repeat units (6-to-1) ranged from 6.3 (Fig. 4b) to 15.7 (Fig. S16†) and depended on the monomer feed. The formation of a copolymer was further confirmed by SEC as monomodal distributions of chains were observed (Đ = 1.39–1.43; Mn = 48.1–46.4 kDa) (Table S1†). Although the copolymer containing a relatively high barrelene content (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 6.3) was found to be soluble in THF, CH2Cl2, and CHCl3, the corresponding solutions slowly became gelatinous and ultimately insoluble when exposed to air.44 For example, signals at 6.4 ppm, 7.1–7.2 ppm, and 7.2–7.4 ppm were identified in the 1H NMR spectrum recorded for a copolymer that was stored for three weeks in an aerated solution of CH2Cl2. The signals were diagnostic of the cis and trans vinylenes as well as the aryl groups in PPV,45 respectively (Fig. 4c), and the molar ratio of 6
1 = 6.3) was found to be soluble in THF, CH2Cl2, and CHCl3, the corresponding solutions slowly became gelatinous and ultimately insoluble when exposed to air.44 For example, signals at 6.4 ppm, 7.1–7.2 ppm, and 7.2–7.4 ppm were identified in the 1H NMR spectrum recorded for a copolymer that was stored for three weeks in an aerated solution of CH2Cl2. The signals were diagnostic of the cis and trans vinylenes as well as the aryl groups in PPV,45 respectively (Fig. 4c), and the molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was found to increase (Fig. S14†). The reduced solubility was attributed to the generation of PPV followed by the physical cross-linking of the conjugated segments via π–π stacking interactions.
1 was found to increase (Fig. S14†). The reduced solubility was attributed to the generation of PPV followed by the physical cross-linking of the conjugated segments via π–π stacking interactions.
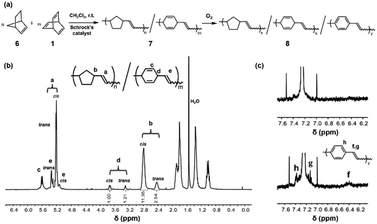
Compared to a freshly prepared copolymer of norbornene and barrelene, the UV/vis data recorded for the material after it was exposed to aerated CH2Cl2 for one month featured a bathochromic absorption at 365 nm (Fig. 5a and b). To drive the oxidation reaction, 7 was treated with DDQ (2.0 equiv. with respect to the barrelene repeat unit) in CH2Cl2 (8.0 mg of copolymer per mL of solvent) for 5 h at room temperature. The UV/vis spectrum recorded for the DDQ-treated copolymer exhibited a strong absorbance at 390 nm which agreed with spectroscopic data reported for PPV.46 The oxidizing conditions also caused the copolymer to fluoresce. For comparison, the material obtained after exposing copolymer 7 to aerated CH2Cl2 for one month exhibited an emission maximum (λem, max) at 460 nm in CH2Cl2 (λex = 365 nm) whereas the DDQ-treated material emitted at 473 nm. Collectively, these data indicated that the barrelene units in the copolymers converted to PPV upon exposure to oxidizing conditions and that stronger oxidants enhanced the conjugation length of the resultant PPV.47
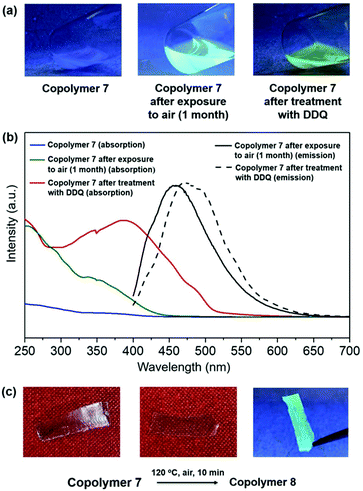
Compared to poly(barrelene), the oxidation of poly(barrelene-co-norbornene) was found to be relatively slow in the solid-state at room temperature which may be due to the inert co-monomer (norbornene). No significant differences were observed in the 1H NMR and Raman spectra recorded for the copolymer after it was stored in air for two months at room temperature (Fig. S15 and S20†). However, heating a film of the copolymer to 120 °C in the air for 10 min caused the material to change to yellow, and the resulting product appeared fluorescent (λem = 461 nm; λex = 365 nm) in the solid-state (Fig. 5c). A series of thermal measurements indicated that the copolymer exhibited an exothermic reaction upon being heating to 100 °C, presumably due to oxidation (dehydrogenation), yet did not experience significant mass loss until approximately 400 °C (under N2) (Fig. S17 and S18†).
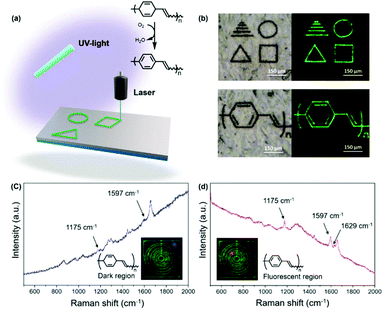
Since exposure of the aforementioned copolymer to elevated temperatures resulted in an optical response, the potential of using the material in DLW applications was explored using a laser capture microdissection (LCM) system (Fig. 6a).48 A 400 nm diameter laser beam at a wavelength of 355 nm was used to write on substrates that consisted of the copolymer at speeds of approximately 350 μm s−1. As shown in Fig. 6b, irradiated areas were found to be fluorescent and the high precision of the laser enabled relatively complex patterns with micrometer-sized dimensions to be written within minutes (Videos S1 and S2†). To determine if the laser facilitated the oxidation of copolymer, the dark and fluorescent regions were investigated by Raman spectroscopy. As shown in Fig. 6c and d, the dark regions exhibited signals that were consistent with poly(barrelene-co-norbornene) whereas signals consistent with PPV and in accord with the data described above were observed in the fluorescent regions.
In conclusion, barrelene (bicyclo[2.2.2]octa-2,5,7-triene) was synthesized using modifications of previously reported routes and characterized, for the first time, by X-ray diffraction analysis of a coordination complex. The homopolymerization of barrelene as well as the copolymerization of barrelene and norbornene using ROMP is also described. The solubility of the barrelene homopolymers was found to depend on the cis to trans ratio of alkenes in its backbone. The homo- and copolymers underwent spontaneous dehydrogenation under air to afford PPV, as determined using a range of spectroscopic techniques. Direct laser writing of the barrelene-containing copolymers was achieved and resulted in thermal aromatization within seconds. An intrinsic advantage of this polymer chemistry is that the monomer could be incorporated into other macromolecular scaffolds and at varying compositions, and thus render a broad range of materials suitable for use in laser machining and contemporary lithography applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Institute for Basic Science (IBS-R019-D1) is acknowledged for support. We thank Dr Da Luo for assistance with recording the Raman data, Mr Jihong Lyu for assistance with recording the TGA and DSC data, Mr Geonhui Park for assistance with recording the HRMS data, and Mr Jinhoe Hur for assistance with using laser capture microdissection system.Notes and references
- A. J. Heeger, Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture), Angew. Chem., Int. Ed., 2001, 40(14), 2591–2611 CrossRef CAS PubMed.
- A. G. MacDiarmid, “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture), Angew. Chem., Int. Ed., 2001, 40(14), 2581–2590 CrossRef CAS PubMed.
- H. Shirakawa, The Discovery of Polyacetylene Film: The Dawning of an Era of Conducting Polymers (Nobel Lecture), Angew. Chem., Int. Ed., 2001, 40(14), 2574–2580 CrossRef PubMed.
- P. Novak, K. Muller, K. S. Santhanam and O. Haas, Electrochemically Active Polymers for Rechargeable Batteries, Chem. Rev., 1997, 97(1), 207–282 CrossRef CAS PubMed.
- J. Xie, P. Y. Gu and Q. C. Zhang, Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries, ACS Energy Lett., 2017, 2(9), 1985–1996 CrossRef CAS.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Light-emitting diodes based on conjugated polymers, Nature, 1990, 347(6293), 539–541 CrossRef CAS.
- K. M. Coakley and M. D. McGehee, Conjugated polymer photovoltaic cells, Chem. Mater., 2004, 16(23), 4533–4542 CrossRef CAS.
- L. M. Dai, B. Winkler, L. M. Dong, L. Tong and A. W. H. Mau, Conjugated polymers for light-emitting applications, Adv. Mater., 2001, 13(12–13), 915–925 CrossRef CAS.
- P. Kar, Doping in Conjugated Polymers, Polymer Science & Technology General, 2013 Search PubMed.
- J. H. Edwards and W. J. Feast, A New Synthesis of Poly(Acetylene), Polymer, 1980, 21(6), 595–596 CrossRef CAS.
- W. J. Feast, J. Tsibouklis, K. L. Pouwer, L. Groenendaal and E. W. Meijer, Synthesis, processing and material properties of conjugated polymers, Polymer, 1996, 37(22), 5017–5047 CrossRef CAS.
- T. M. Swager, D. A. Dougherty and R. H. Grubbs, Strained Rings as a Source of Unsaturation – Polybenzvalene, a New Soluble Polyacetylene Precursor, J. Am. Chem. Soc., 1988, 110(9), 2973–2974 CrossRef CAS.
- J. Seo, S. Y. Lee and C. W. Bielawski, Unveiling a Masked Polymer of Dewar Benzene Reveals trans-Poly(acetylene), Macromolecules, 2019, 52(8), 2923–2931 CrossRef CAS.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Electroluminescence in conjugated polymers, Nature, 1999, 397(6715), 121–128 CrossRef CAS.
- T. A. Skotheim, R. L. Elsenbaumer and J. R. Reynolds, Handbook of Conducting Polymers, CRC Press, New York, 2nd edn, 1997 Search PubMed.
- T. Junkers, J. Vandenbergh, P. Adriaensens, L. Lutsen and D. Vanderzande, Synthesis of poly(p-phenylene vinylene) materials via the precursor routes, Polym. Chem., 2012, 3(2), 275–285 RSC.
- H. G. Gilch and W. L. Wheelwright, Polymerization of α-halogenated p-xylenes with base, J. Polym. Sci., Part A-1: Polym. Chem., 1966, 4(6), 1337–1349 CrossRef CAS.
- R. W. Lenz, C.-C. Han, J. Stenger-Smith and F. E. Karasz, Preparation of poly(phenylene vinylene) from cycloalkylene sulfonium salt monomers and polymers, J. Polym. Sci., Part A: Polym. Chem., 1988, 26(12), 3241–3249 CrossRef CAS.
- S. Son, A. Dodabalapur, A. J. Lovinger and M. E. Galvin, Luminescence Enhancement by the Introduction of Disorder into Poly(p-phenylene vinylene), Science, 1995, 269(5222), 376–378 CrossRef CAS PubMed.
- M. Van Der Borght, D. Vanderzande and J. Gelan, Synthesis of high molecular weight poly(4,4′-bisphenylene vinylene) and poly(2,6-naphthalene vinylene) via a non-ionic precursor route, Polymer, 1998, 39(17), 4171–4174 CrossRef.
- R. A. Wessling, The Polymerization of Xylylene Bisdialkyl Sulfonium Salts, J. Polym. Sci., Polym. Symp., 1985, 72(72), 55–66 CAS.
- Y. J. Miao and G. C. Bazan, Paracyclophene Route to Poly(P-Phenylenevinylene), J. Am. Chem. Soc., 1994, 116(20), 9379–9380 CrossRef CAS.
- N. Zaquen, L. Lutsen, D. Vanderzande and T. Junkers, Controlled/living polymerization towards functional poly(p-phenylene vinylene) materials, Polym. Chem., 2016, 7(7), 1355–1367 RSC.
- V. P. Conticello, D. L. Gin and R. H. Grubbs, Ring-Opening Metathesis Polymerization of Substituted Bicyclo[2.2.2]Octadienes – a New Precursor Route to Poly(1,4-Phenylenevinylene), J. Am. Chem. Soc., 1992, 114(24), 9708–9710 CrossRef CAS.
- L. Pu, M. W. Wagaman and R. H. Grubbs, Synthesis of poly(1,4-naphthylenevinylenes): Metathesis polymerization of benzobarrelenes, Macromolecules, 1996, 29(4), 1138–1143 CrossRef CAS.
- M. W. Wagaman and R. H. Grubbs, Synthesis of organic and water soluble poly(1,4-phenylenevinylenes) containing carboxyl groups: Living ring-opening metathesis polymerization (ROMP) of 2,3-dicarboxybarrelenes, Macromolecules, 1997, 30(14), 3978–3985 CrossRef CAS.
- M. H. Bitarafan, S. Suomala and J. Toivonen, Sub-microwatt direct laser writing of fluorescent gold nanoclusters in polymer films, Opt. Mater. Express, 2020, 10(1), 138–148 CrossRef CAS.
- I. Diez and R. H. Ras, Fluorescent silver nanoclusters, Nanoscale, 2011, 3(5), 1963–1970 CAS.
- P. Kunwar, J. Hassinen, G. Bautista, R. H. Ras and J. Toivonen, Direct laser writing of photostable fluorescent silver nanoclusters in polymer films, ACS Nano, 2014, 8(11), 11165–11171 CAS.
- O. I. Avila, J. M. P. Almeida, F. R. Henrique, R. D. Fonseca, G. F. B. Almeida, D. T. Balogh and C. R. Mendonça, Femtosecond-laser direct writing for spatially localized synthesis of PPV, J. Mater. Chem. C, 2017, 5(14), 3579–3584 RSC.
- J. Hine, J. A. Brown, L. H. Zalkow, W. E. Gardner and M. Hine, The Synthesis of Bicyclo [2,2,2]-2,5-octadiene1,2, J. Am. Chem. Soc., 1955, 77(3), 594–598 CrossRef CAS.
- H. E. Zimmerman and R. M. Paufler, J. Am. Chem. Soc., 1960, 82, 1514 CrossRef CAS.
- H. E. Zimmerman, G. L. Grunewald, R. M. Paufler and M. A. Sherwin, Synthesis and physical properties of barrelene, a unique Moebius-like molecule, J. Am. Chem. Soc., 1969, 91(9), 2330–2338 CrossRef CAS.
- S. I. Kozhushkov, T. Preuß, D. S. Yufit, J. A. K. Howard, K. Meindl, S. Rühl, C. Yamamoto, Y. Okamoto, P. R. Schreiner, B. C. Rinderspacher and A. de Meijere, 4,7,11-Triheterotrishomocubanes – Propeller-Shaped Highly Symmetrical Chiral Molecules Derived from Barrelene, Eur. J. Org. Chem., 2006,(11), 2590–2600 CrossRef CAS.
- S. Cossu, S. Battaggia and O. DeLucchi, Barrelene, a new convenient synthesis, J. Org. Chem., 1997, 62(12), 4162–4163 CrossRef CAS.
- D. M. Matias and J. S. Johnson, Synthesis and Desymmetrization of meso Tricyclic Systems Derived from Benzene Oxide, J. Org. Chem., 2018, 83(8), 4859–4866 CrossRef CAS PubMed.
- U. Koelle and U. Englert, Crystal structure of di-μ-chloro-bis(η4-norbornadienerhodium), (C7H8Rh)2Cl2, Cryst. Mater., 2010, 211(1), 64 Search PubMed.
- R. B. Taylor and P. W. Jennings, Solvent Effects on the Valence Isomerization Catalyst (Norbornadiene)Rhodium Chloride Dimer, Inorg. Chem., 1981, 20(11), 3997–3999 CrossRef CAS.
- Polymerization was not observed when 1 was treated with a series of Ru-based catalysts (e.g., G1, G2, or G3). As such, attention was re-directed toward a Mo-based catalyst that was previously shown to polymerize barrelene derivatives (see: ref. 25 and 26).
- D. M. Byler, Y. Patel and G. A. Arbuckle-Keil, An IR study of poly-1,4-phenylenevinylene (PPV), the 2,5-dimethoxy derivative [(MeO)2-PPV], and their corresponding xanthate precursor polymers and monomers, Spectrochim. Acta, Part A, 2011, 79(1), 118–126 CrossRef PubMed.
- J. Chen, P. Yang, C. Wang, S. Zhan, L. Zhang, Z. Huang, W. Li, C. Wang, Z. Jiang and C. Shao, Ag nanoparticles/PPV composite nanofibers with high and sensitive opto-electronic response, Nanoscale Res. Lett., 2011, 6(1), 121 CrossRef PubMed.
- Y. Furukawa, A. Sakamoto and M. Tasumi, Raman and Infrared Studies on the Molecular-Structures of Poly(1,4-Phenylenevinylene) and Poly(2,5-Thienylenevinylene), J. Phys. Chem., 1989, 93(14), 5354–5356 CrossRef CAS.
- L. B. W. Lee and R. A. Register, Hydrogenated ring-opened polynorbornene: A highly crystalline atactic polymer, Macromolecules, 2005, 38(4), 1216–1222 CAS.
- For comparison, copolymers with relatively high norbornene contents (6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 15.7) appeared to be relatively stable and remained soluble over extended periods of time.
1 = 15.7) appeared to be relatively stable and remained soluble over extended periods of time. - F. Wang, F. He, Z. Xie, M. Li, M. Hanif, X. Gu, B. Yang, H. Zhang, P. Lu and Y. Ma, A solution-processible poly(p-phenylene vinylene) without alkyl substitution: Introducing the cis-vinylene segments in polymer chain for improved solubility, blue emission, and high efficiency, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(15), 5242–5250 CrossRef CAS.
- J. I. Jin, Y. H. Lee and H. K. Shim, Synthesis and characterization of poly(2-methoxy-5-nitro-1,4-phenylenevinylene) and poly(1,4-phenylenevinylene-co-2-methoxy-5-nitro-1,4-phenylenevinylenes), Macromolecules, 1993, 26(8), 1805–1810 CrossRef CAS.
- Y. N. Li, G. Vamvounis and S. Holdcroft, Control of conjugation length and enhancement of fluorescence efficiency of poly(p-phenylenevinylene)s via post-halogenation, Chem. Mater., 2002, 14(3), 1424–1429 CrossRef CAS.
- Improved resolutions were observed when the copolymer with a relatively high norbornene content (6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 15.7) was used as the substrate, presumably due to the higher transparency of the corresponding films. The ESI contains data (Fig. S21†) that were recorded after DLW on substrates comprised of copolymers with relatively low norbornene contents (6
1 = 15.7) was used as the substrate, presumably due to the higher transparency of the corresponding films. The ESI contains data (Fig. S21†) that were recorded after DLW on substrates comprised of copolymers with relatively low norbornene contents (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 6.3) for comparison.
1 = 6.3) for comparison.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1585233. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0py00869a |
| This journal is © The Royal Society of Chemistry 2020 |