High chemical recyclability of vinyl lactone acrylic bioplastics†
Abstract
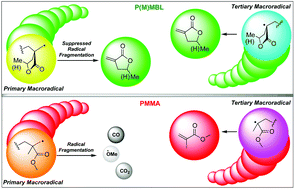
The depolymerization selectivity of poly(methyl methacrylate) (PMMA) under thermolysis and dynamic vacuum is inherently limited by the repeat-unit structure, leaving much (∼47%) of PMMA to fragment and carbonize to char. Here we show that renewable, high-performance alternatives to PMMA with vinyl lactone repeat units, poly(α-methylene-γ-butyrolactone) (PMBL) and poly(γ-methyl-α-methylene-γ-butyrolactone) (PγMMBL), unexpectedly depolymerize much more selectively and recover monomers with considerably higher yield and purity than PMMA (76% pure monomer isolated from PγMMBL) and are also devoid of char formation, leaving the residue as only the oligomers with a total mass balance. To uncover the origin of the unexpected high chemical recyclability of P(M)MBL, this study has ascertained their ceiling temperature (Tc) by density functional theory, trapped and analyzed both primary and tertiary macroradicals generated during the depolymerization, and probed the stability of macroradicals using Lewis acid additives and mixed plastic feeds. The evidence obtained through this study suggests that the much enhanced recyclability of P(M)MBL bioplastics relative to PMMA is not due to their differences in Tc values, but rather the linear ester and cyclic ester-imparted difference in stability and monomer-production roles of primary and tertiary macroradicals generated in the random chain scission processes.
- This article is part of the themed collection: Plastics in a circular economy


 Please wait while we load your content...
Please wait while we load your content...