Open Access Article
Open Access ArticleAccessible and sustainable Cu(0)-mediated radical polymerisation for the functionalisation of surfaces†
Oliver Frank
Uttley
 *a,
Leonie Alice
Brummitt
*a,
Leonie Alice
Brummitt
 a,
Stephen David
Worrall
a,
Stephen David
Worrall
 b and
Steve
Edmondson
b and
Steve
Edmondson
 a
a
aDepartment of Materials, School of Natural Sciences, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: oliver.uttley@manchester.ac.uk
bAston Institute of Materials Research, School of Engineering and Applied Science, Aston University, Birmingham, B4 7ET, UK
First published on 19th May 2020
Abstract
Polymer brushes have great potential for use in functionalising surfaces due to their chemical and mechanical robustness, and wide variety of useful properties including antibacterial and antifouling behaviour. One such grafted polymer of interest is poly[2-(methacryloyloxy)ethyl]trimethylammonium chloride (PMETAC), shown to have excellent antibacterial behaviour due to the presence of quaternary ammonium chloride groups (QACs). Previous studies have shown that increasing the density of QACs increases the efficacy of these surfaces, therefore the production of thick PMETAC brushes is highly desirable. Cu(0)-mediated radical polymerisation (CuRP) offers a simple route to the production of these surfaces. A movement towards more sustainable chemistry has led to research into polymerisations in environmentally benign solvent, with focus placed on recycled and easily accessible catalysts. In this study, the growth of PMETAC brushes up to 300 nm dry thickness (∼![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 nm water-swollen thickness) is demonstrated, thicker than any previous report we have found for this polymer brush. Furthermore, tap water is used as a cheap and readily available solvent, with a catalyst derived from copper wire. The use of copper wire, compared to the commonly used CuBr2 catalyst, leads to thicker coatings which also display a lower swelling ratio, implying an increased grafting density. The protocol can be continuously cycled over a 7-day period without changing the monomer solution or catalyst, with numerous wafers being functionalised over the time period with no significant reduction in grafted amount. In addition, the polymerisation can be carried out in ambient (non-inert) conditions with no degassing steps, again without with significant detriment to grafting.
425 nm water-swollen thickness) is demonstrated, thicker than any previous report we have found for this polymer brush. Furthermore, tap water is used as a cheap and readily available solvent, with a catalyst derived from copper wire. The use of copper wire, compared to the commonly used CuBr2 catalyst, leads to thicker coatings which also display a lower swelling ratio, implying an increased grafting density. The protocol can be continuously cycled over a 7-day period without changing the monomer solution or catalyst, with numerous wafers being functionalised over the time period with no significant reduction in grafted amount. In addition, the polymerisation can be carried out in ambient (non-inert) conditions with no degassing steps, again without with significant detriment to grafting.
1. Introduction
Due to their excellent mechanical and chemical robustness, polymer brushes, densely surface-tethered polymer chains, offer great potential for the functionalisation of surfaces for a broad range of applications including antifouling,1,2 lubrication,3,4 and thermo-responsive surfaces.5 To date this type of functional surface coating has not been widely exploited, due to many applicable polymerisation methods being limited to small substrates and low-throughput batch processes due to inert atmosphere requirements of these oxygen sensitive chemistries. Poly[2-(methacryloyloxy) ethyl]trimethylammonium chloride (PMETAC) brushes have shown excellent antifouling and antibacterial properties in previous studies.1,2 These properties are thought to derive from the cationic quaternary ammonium chloride groups (QACs), with antifouling and antibacterial properties increasing with greater polymer thickness and so charge density.6,7 An accessible polymerisation route to thick PMETAC brushes, and so an increased surface density of QACs, is therefore of great interest.When producing functionalised surfaces, especially those for biomedical applications, it is important to consider the synthesis procedure in order to reduce any hazards associated with both process and product, since solvent choice affects safety of both product and process, and production cost.8 Water is a safe, environmentally benign and abundant solvent and is considered the “gold standard” solvent choice for reducing the environmental impact of industrial processes.8,9 However, often water is required to be extensively purified, with deionized and ultrapure water used in many processes. The use of purified water is costly, and the energy associated with purification increases environmental impact.8
In this paper, we demonstrate a process for synthesising antibacterial polymer brush coatings with unpurified tap water as solvent. Furthermore, we demonstrate recycling of the catalyst and polymerisation solution for multiple substrates (indeed in theory also compatibility with continuous processes such as roll-to-roll), making our process attractive for scale-up of brush synthesis with reduced costs, increased efficiency, and decreased hazards and environmental impacts compared to many existing procedures.
Various methods for the growth of polymer brushes have been detailed in previous work with reversible addition–fragmentation chain transfer (RAFT)10,11 and atom transfer radical polymerization (ATRP)12,13 being most popular due to both exhibiting excellent control over molecular weight, polydispersity and chain functionality. However, both have limitations: the chain transfer agents required for control in RAFT have limited commercial availability and relatively high cost.14 ATRP, although a well-defined and controlled polymerisation,15 can be highly oxygen sensitive, and is often carried out in undesirable or harmful solvents.8 In 2007 Matyjaszewski et al.,16 reported on activators regenerated by electron transfer atom transfer radical polymerization (ARGET ATRP) for the growth of polymer brushes, a method which offers a range of advantages over conventional ATRP including: reduced amounts of both metal catalyst and solubilising ligand; no addition of oxygen-sensitive and expensive Cu(I) catalysts due to it being formed in situ due to an addition of excess reducing agent; reduced oxygen sensitivity, with the reaction being able to proceed in ambient atmosphere. The risk of failure of the reaction is often decreased when an attempt to deoxygenate the solution is made.16 These factors make ARGET ATRP more cost effective, environmentally friendly and safer than ATRP, making it desirable for the use of large-scale polymer brush synthesis, particularly in industry. ARGET ATRP has shown good results in the synthesis of various polymer brushes,16–19 however, we were unable to find prior examples of the synthesis of PMETAC brushes via this route.
Recently, Mendonça et al.,8 successfully showed the use of untreated water as solvent for solution-phase ATRP and ARGET ATRP. The synthesis of poly(ethylene glycol) methyl ether acrylate chains in solution was detailed, with kinetics and molecular weight obtained very similar for reactions in both ultrapure and untreated water. This research showed a more eco-friendly and sustainable solution polymerisation, when compared to other forms of ARGET ATRP. Extending this work to surface-initiated polymerisations to grow polymer brushes with unpurified water solvent while greatly reducing Cu(II) halide waste could be a significant step forward towards the cost effective and safe functionalisation of surfaces.
The use of Cu(0) based catalysts, such as copper wire, for polymer synthesis, has been documented before.20–22 Copper wire is an excellent source of catalyst due to its ease of preparation, high observed reaction rates, predictability, tunability and importantly, reusability.23 Zhang et al.,24,25 previously detailed the growth of PMETAC brushes via surface initiated Cu(0) controlled radical polymerisation (Si-CuCRP). This procedure used recycled and readily available forms of copper as a catalyst (plates and coins), with a minimal amount of solution required for the polymerisation to proceed. The method showed excellent oxygen tolerance, and the polymerisation allowed for high grafting densities to be achieved. The copper plate was also reused for repeated reactions.
We detail the growth of PMETAC brushes, the thickest we have been able to find in literature, via an efficient and accessible aqueous Cu(0)-mediated radical polymerisation (CuRP) method based on a previous ARGET ATRP process, in which both the solvent and copper catalyst are abundant. Our method offers a low cost and efficient route to functionalising surfaces with thick cationic PMETAC brushes, which are ideal candidates for antibacterial surfaces. Both the reduction of solvent waste and reuse of reaction mixture are attractive for scale-up. We detail a method of reusing both the catalyst and monomer solution for a number of cycles to reduce solvent and metal catalyst waste. Our PMETAC brush polymerisation can be conducted in n ambient atmosphere with no deoxygenation steps required, significantly reducing experimental set up times and costs associated with purging reaction vessels. Interestingly, thicker PMETAC brushes are grown in ambient atmosphere, compared to an inert atmosphere, and we propose a secondary initiator present in the solution which may modify reaction kinetics.
2. Experimental section
2.1 Materials
Silicon wafers (〈100〉 orientation, boron-doped, 0–100 Ω cm) were purchased from PI-KEM (Tamworth, UK). Hydrogen peroxide (≥35 wt%) was supplied by VWR. 35 wt% ammonia solution (analytical reagent grade), ethanol, methanol (HPLC gradient grade), propan-2-ol, and cellulose dialysis bags (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecular weight cut-off (MWCO)) were supplied by Fisher Scientific (Leicestershire, UK). (3-Aminopropyl)triethoxysilane (≥98%), trimethylamine (laboratory reagent grade), tetrahydrofuran (HPLC reagent grade, ≥99.9%), 4 Å molecular sieves (8–12 mesh), 2-bromoisobutyryl bromide (98%), propionyl bromide (95%), [2-(methacryloyloxy)ethyl] trimethylammonium chloride solution (80 wt% in H2O), copper(II) bromide (99%), copper wire (d = 1 mm, ≥99.9%), 2,2′-bipyridyl, ascorbic acid (99%) and deuterium oxide (99.9%) were supplied by Sigma Aldrich (St Louis, USA). Tap water was supplied from United Utilities, Manchester. Typical ion content as reported by supplier: calcium = 10.2 mg L−1; chloride = 5.8 mg L−1; magnesium = 1.21 mg L−1; pH = 7.22; sodium = 5.23 mg L−1; sulphate = 8.34 mg L−1; hardness as calcium carbonate = 30 mg L−1.
000 molecular weight cut-off (MWCO)) were supplied by Fisher Scientific (Leicestershire, UK). (3-Aminopropyl)triethoxysilane (≥98%), trimethylamine (laboratory reagent grade), tetrahydrofuran (HPLC reagent grade, ≥99.9%), 4 Å molecular sieves (8–12 mesh), 2-bromoisobutyryl bromide (98%), propionyl bromide (95%), [2-(methacryloyloxy)ethyl] trimethylammonium chloride solution (80 wt% in H2O), copper(II) bromide (99%), copper wire (d = 1 mm, ≥99.9%), 2,2′-bipyridyl, ascorbic acid (99%) and deuterium oxide (99.9%) were supplied by Sigma Aldrich (St Louis, USA). Tap water was supplied from United Utilities, Manchester. Typical ion content as reported by supplier: calcium = 10.2 mg L−1; chloride = 5.8 mg L−1; magnesium = 1.21 mg L−1; pH = 7.22; sodium = 5.23 mg L−1; sulphate = 8.34 mg L−1; hardness as calcium carbonate = 30 mg L−1.
2.2 Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL was added via syringe to cover the strip in the sample tube. Nitrogen pressure was applied to the tube throughout the procedure. 0.25 mL of 2-bromoisobutyryl bromide (BIBB) was then added for every 10 mL of tetrahydrofuran. The solution was left to react for 1 hour. The solution was removed, before tubes and silicon strips were washed with a series of 30 mL tetrahydrofuran and 30 mL methanol. The strips were then removed carefully with tweezers, rinsed with deionised water and dried with nitrogen. Successful initiator deposition was observed as a change in surface energy, with samples changing from hydrophilic to hydrophobic.
1 mL was added via syringe to cover the strip in the sample tube. Nitrogen pressure was applied to the tube throughout the procedure. 0.25 mL of 2-bromoisobutyryl bromide (BIBB) was then added for every 10 mL of tetrahydrofuran. The solution was left to react for 1 hour. The solution was removed, before tubes and silicon strips were washed with a series of 30 mL tetrahydrofuran and 30 mL methanol. The strips were then removed carefully with tweezers, rinsed with deionised water and dried with nitrogen. Successful initiator deposition was observed as a change in surface energy, with samples changing from hydrophilic to hydrophobic.
2.3 Analysis
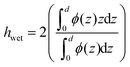 | (1) |
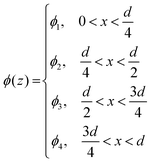 | (2) |
3. Results and discussion
3.1. Surface initiated ARGET ATRP and CuRP in water
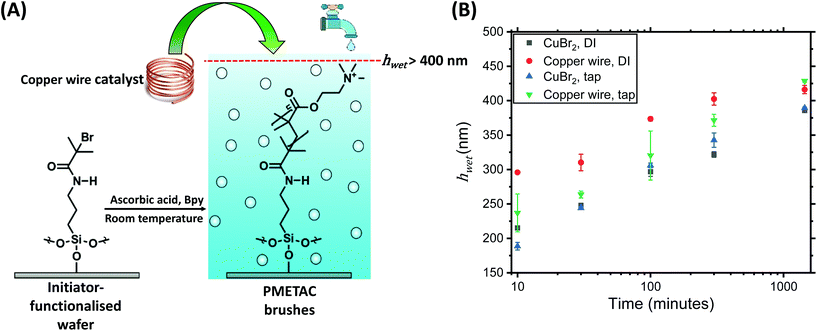
Before studying the effectiveness of a copper wire as a source of catalyst, it is first important to look at the growth of PMETAC brushes via a more typical method. The effectiveness of both activators regenerated by electron transfer atom transfer radical polymerisation (ARGET ATRP), using a copper(II) halide catalyst, and Copper(0)-mediated radical polymerisation (CuRP)(Fig. 1A), using copper wire as a source of catalyst were compared. Further to this, the solvent was also investigated, with deionised (purified) and tap (non-purified) waters being assessed.Ion chromatography was performed to analyse chloride and bromide ion content of both water sources. A chloride and bromide ion content of 0.13 mg L−1 and <0.05 mg L−1, respectively, was observed for DI water. Tap water had a chloride and bromide ion content of 7.56 mg L−1 and <0.05 mg mL−1, respectively, in line with quoted values from the utilities company.
For all reactions, aqueous solutions of [2-(methacryloyloxy)ethyl] trimethylammonium chloride (METAC), 2,2′-bipiridyl (Bpy) and ascorbic acid were made with a molar ratio of 3400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. For ARGET ATRP reactions with copper(II) halide, a molar ratio of 1
1, respectively. For ARGET ATRP reactions with copper(II) halide, a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 for ascorbic acid to CuBr2 was used. For CuRP, a coiled piece of copper wire (l = 10 cm, d = 1 mm) was used. All reactions were carried out at ambient temperature in a nitrogen atmosphere. It is important to note that due to the ionic structure of METAC with a Cl− counterion, the addition of an extra halide salt to improve control is not needed, as noted in previous work.8 Polymer growth kinetics are shown in Fig. 1B.
0.1 for ascorbic acid to CuBr2 was used. For CuRP, a coiled piece of copper wire (l = 10 cm, d = 1 mm) was used. All reactions were carried out at ambient temperature in a nitrogen atmosphere. It is important to note that due to the ionic structure of METAC with a Cl− counterion, the addition of an extra halide salt to improve control is not needed, as noted in previous work.8 Polymer growth kinetics are shown in Fig. 1B.
The results show that replacing deionized water (DI) with tap water has no detrimental effect on the growth of PMETAC brushes, and so tap water is suitable for use in both ARGET ATRP and CuRP in future work, successfully translating results from Mendonça et al.,8 on solution polymerization to the surface initiated growth of polymer brushes. This finding should be of benefit for scale-up and industrial application of these brushes, with no requirement for solvent or reagent purification in order for the growth of polymer brushes to proceed efficiently.
CuBr2 and other copper(II) halide compounds are often used for the catalyst complex in ARGET ATRP,16 however, these compounds lead to the production of halogen-containing waste which can be difficult to properly and safely dispose of.32,33 The use of copper wire as a source of catalyst for reactions has been detailed before,20,34 however, importance was placed on control of molecular weight control and polydispersity of chains grown in solution. Further to this, prior work has not applied an environmentally benign solvent, such as H2O.
Our results (Fig. 1B) clearly demonstrate the growth of thicker PMETAC brushes (up to 30–40 nm greater wet thickness at each time step), when using copper wire catalysed CuRP compared with CuBr2 catalysed ARGET. A maximum dry and wet thickness of 300 nm and 428.7 nm was observed, respectively, from CuRP as shown in Table 1. To our knowledge these are the thickest PMETAC brushes grown in the current literature, with previous work by Zhang et al.,24,25 detailing a method of growing PMETAC brushes with a dry thickness of ∼230 nm via surface-initiated – Cu(0)-mediated controlled radical polymerisation (SI-CuCRP). PMETAC and other polycationic brushes have shown excellent antibacterial behaviour due to the presence of charged QAC groups.1,35 A key advantage both our work and the work by Zhang et al.,24,25 was that no copper halide catalyst was required. Copper wire is a readily available, easily accessible and reusable alternative to compounds such as CuBr2 in controlled radical polymerisations. Our results show a clear step forward towards the production of thick PMETAC brush functionalised surfaces via a low energy, cost effective and green polymerisation.
| Catalyst | h dry (nm) | h wet (nm) | Swelling ratio (SR) | R a (nm) | R q (nm) | |
|---|---|---|---|---|---|---|
| DI | CuBr2 (ARGET) | 179.8 ± 1.0 | 385.6 ± 1.4 | 2.14 | 1.19 ± 0.39 | 1.95 ± 0.27 |
| Tap | CuBr2 (ARGET) | 187.2 ± 0.7 | 389.4 ± 0.6 | 2.08 | 0.91 ± 0.20 | 1.37 ± 0.21 |
| DI | Copper wire (CuRP) | 300.1 ± 19.3 | 416.2 ± 3.0 | 1.39 | 2.25 ± 0.23 | 4.26 ± 0.87 |
| Tap | Copper wire (CuRP) | 282.3 ± 4.9 | 428.6 ± 0.9 | 1.52 | 1.36 ± 0.04 | 2.00 ± 0.04 |
Although we have shown growth of thicker PMETAC brushes than previously, it is important to additionally assess both the swelling ratio (SR) and roughness of brushes in order to assess the grafting density and the homogeneity of surfaces. Table 1 shows the swelling ratio of brushes which is calculated as hwet/hdry. Both the average roughness (Ra) and root mean square roughness (Rq) were calculated from 2500 μm2 scans of the surface via AFM, as a means of measuring homogeneity. Representative images can be found in the ESI (Fig. S2†).
Recently Oh et al.,2 attempted to estimate the grafting density (σ) (defined here as chains nm−2) of PMETAC brushes, however, their calculation was derived from a scaling law for neutral brushes:36hwet ∝ Nσ⅓, where N is the degree of polymerization. We believe this approach is not valid and instead, any estimation of σ for PMETAC should begin with the predicted scaling law for strong polyelectrolyte brushes in the osmotic regime, where wet thickness is independent of grafting density:36,37
| hwet ∝ Nσ0. | (3) |
When brushes, either charged or neutral are in the dry state, thickness is directly proportional to grafting density:38
| hdry ∝ Nσ1. | (4) |
If swelling ratio (SR) is defined as the wet thickness divided by the dry thickness for one sample (i.e. with the same degree of polymerization, N), we can relate grafting density to swelling ratio, for brushes of the same monomer structure:
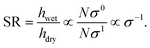 | (5) |
Therefore, differences in SR between samples of strong polyelectrolyte brushes with the same structure should provide a good estimate of the difference in σ, being inversely proportional to SR.
From Table 1 it is clear to see that polymerisations containing copper wire as a catalyst source (CuRP) led to lower SR and hence a higher grafting density being achieved than with ARGET. Further to this, water type has little influence on SR, further confirming that tap water is suitable for use as a solvent in these polymerizations. However, the use of copper wire as a catalyst leads to higher values of both Ra and Rq. The increase in roughness values may simply be due to the increase in overall brush thickness, with the roughness as a percentage of dry brush thickness being similar for all samples (∼0.5%). It was noted that the use of a copper wire catalyst led to visibly less homogenous sample surfaces with variation in thin-film colours being seen across the silicon wafer, with samples grown using a CuBr2 catalyst showing a uniform gold thin-film colour over each ∼1 cm2 sample.
3.2. Cu(0)-mediated radical polymerisation
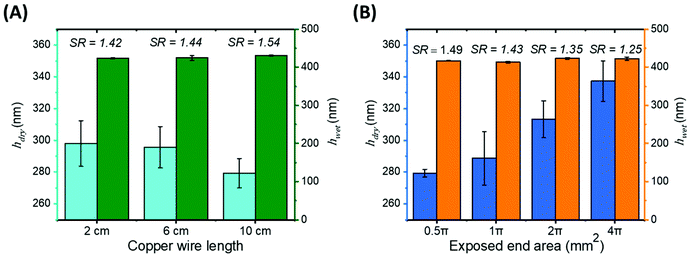
Interestingly, during reactions it was observed that leaching of a red colour was seen from each end of the copper wire, presumed to be the dissolution of copper ions. The effect of both the length and number of exposed ends of copper wire on the growth over a 24-hour period was investigated (Fig. 2). Previous work by Magenau et al.,20 investigated the effect of copper wire on reaction kinetics of methyl methacrylate (MMA) via ATRP, with rate dependent and increasing with surface area of copper wire, and thus copper catalyst concentration. However, copper wire was used with a small amount of CuBr2 in this prior work, in order to gain better control of polydispersity and reaction kinetics, and the reaction was a solution polymerisation of MMA chains.In our work, the length of copper wire is seen to have no significant effect on the swollen brush thickness (and so degree of polymerization) with a small increase in swelling ratio observed with wire length (Fig. 2A).
In order to change the number of exposed ends, a 10 cm length of copper wire was cut into equal sizes (i.e. 8 ends = 2.5 cm lengths). The exposed end area was calculated as the number of ends multiplied by πr2 (r = 0.5 mm). Remarkably, with increasing exposed end area all growths produced brushes with near-identical wet thickness (∼425 nm) suggesting near-identical molecular weight, while smoothly increasing dry thickness and so grafting density. Controlling grafting density independently of molecular weight is highly desirable in polymer brush fabrication, allowing surface properties to be more precisely tailored and investigated. The tuning of Cu(0) ion concentration, through the simple method of cutting wire into more pieces, is therefore a powerful tool for precise surface grafting.
It is likely that due to the increased exposed end area, more copper ions can dissolve at the beginning of the reaction, meaning more catalyst complex is present, allowing for a higher early rate of initiation. The polymerisation can therefore occur from more active initiator sites, increasing the grafting density. Previous studies on copper mediated ATRP of PMETAC brushes have shown that altering catalyst concentration allows for the control of grafting density.39 Magenau et al.,20 showed that increasing the surface area of copper wire as a catalyst source in the solution ATRP of MMA lead to increased initial reaction rates, agreeing with our results. It is likely that significant copper dissolution only occurs at the freshly-cut ends of the wire due to lack of a protective oxidised layer. This slow release of copper ions from the exposed ends allows for sensitive tuning of the initial reaction rate, thus allowing for increased initiation, and in turn increasing grafting density. To a first approximation, the degree of polymerisation in a surface-initiated controlled radical polymerisation should not depend on either initiator concentration or catalyst concentration, as observed in our experiments.
3.3. Ambient atmosphere Cu(0)-mediated radical polymerisation
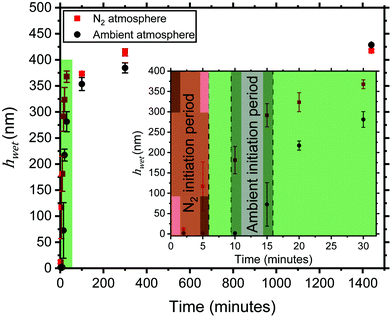
To further probe the robustness and sustainability of the CuRP system, polymerizations were carried out in ambient atmosphere with all deoxygenation steps removed. Commonly in Cu-catalysed polymerisations, an attempt to thoroughly deoxygenate the solution is made, and polymerisation is carried out under an inert atmosphere in order to improve polymerisation consistency and extent.8,34,40 However deoxygenation procedures are costly, time consuming and rely on access to specialised equipment, such as Schlenk lines.22Methods to reduce oxygen sensitivity and oxygen content within a reaction, such as the addition of a reducing agent,16,41 the use of a glucose oxidase enzyme (GOx),42 and limiting headspace,22 have been employed to tackle this problem. To reduce the oxygen sensitivity of our procedure, excess ascorbic acid was added as an oxygen scavenger, due to it being low cost, easily-obtainable and non-toxic. The kinetics for reactions in both ambient and nitrogen atmospheres are shown in Fig. 3.
Over the 24-hour growth period, PMETAC brushes grew to a very similar thickness in both atmospheres, with no significant difference in dry thickness observed (Table 2). The swelling ratio was also identical, indicating the same grafting density was achieved.
| Atmosphere | h dry (nm) | h wet (nm) | Swelling ratio (SR) | R a (nm) | R q (nm) |
|---|---|---|---|---|---|
| Nitrogen | 282.3 ± 4.9 | 417.4 ± 2.9 | 1.52 | 1.36 ± 0.04 | 2.00 ± 0.04 |
| Ambient | 274.7 ± 8.1 | 428.6 ± 0.9 | 1.52 | 1.93 ± 0.67 | 2.56 ± 0.71 |
During the polymerisation time, it was observed that the solution exposed to oxygen in ambient atmosphere became more viscous (ESI, Fig. S3†). It was also noted that an inhibition period was observed during the first 10 minutes of the reaction in ambient atmosphere (Fig. 3 inset), with little growth of PMETAC brushes observed. Both the increase in viscosity of the polymerisation solution and inhibition period are indicative of a secondary reaction in solution. 1H NMR spectroscopy of the polymerisation solution from both atmospheres was carried out in order to identify a possible secondary polymerisation (Fig. 4).
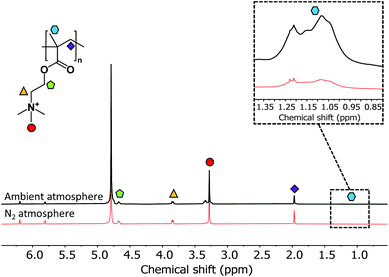 | ||
| Fig. 4 1H NMR spectra taken for both METAC solution that was degassed and used under nitrogen pressure and solution that was not degassed and used in ambient conditions after 24 hours. | ||
A broad peak between 1.3 and 0.9 ppm was observed for samples from both atmospheres, indicative of a methacrylic backbone, consistent with previous literature.43 Furthermore, spectra displayed other peaks corresponding to PMETAC chains in solution, assigned according to literature.43 Although it is possible that the NMR spectra observed is due to chains de-grafting from the silicon surface rather than secondary polymerisation occurring in solution, the polymer concentration produced by degrafting is likely to be far below the sensitivity of NMR, even if complete degrafting is assumed.
Previous work by Reyhani et al.,44 showed a method of initiating a RAFT polymerisation using a Fenton reaction. Fe2+ ions were used to generate hydroxyl radicals from hydrogen peroxide, allowing for initiation. They found that there was an induction period during which oxygen is preferentially consumed, followed by a well-controlled and rapid polymerisation.45
Although no H2O2 is present in, or added to, our reaction it is likely that the presence of Cu(I) ions can lead to hydroxyl radical production, from any oxygen and H2O present.15 This has been observed in previous literature, where a reaction between glucose, glucose oxidase and oxygen with Cu(I) species leading to the production of hydroxyl radicals, which acted as an initiating species.46 It is important to note that our reactions contained a small amount of ascorbic acid. Previous studies have shown the formation of hydroxyl radicals and H2O2 from the oxidation of ascorbic acid by oxygen in copper catalysed reactions.47,48 It is likely that a combination of these reactions is allowing for the formation of radicasl, and therefore, the initiation of the solution polymerisation.
To further validate that a secondary polymerisation was occurring and initiating from non-grafted species, CuRP reaction mixtures were made as above, and left for 24 hours without addition of an initiator-coated silicon wafer. If the polymerisation is surface-confined, no polymerization should occur, and the monomer solution should remain unchanged. 1H NMR data can be found in the ESI (Fig. S4†). NMR shows a distinct peak between 1.3 and 0.9 ppm for both oxygenated and deoxygenated samples confirming that a secondary polymerisation is indeed occurring. Even for deoxygenated monomer solutions it is likely that a small amount of oxygen remains, with the NMR peaks present with much lower intensity. The growth of PMETAC brushes via CuRP in ambient conditions offers a robust route to the synthesis of thick PMETAC brushes, however, there may be implications for efficiency and repeatability due to secondary polymerisation in solution.
3.4. Multiple cycle Cu(0)-mediated radical polymerisation
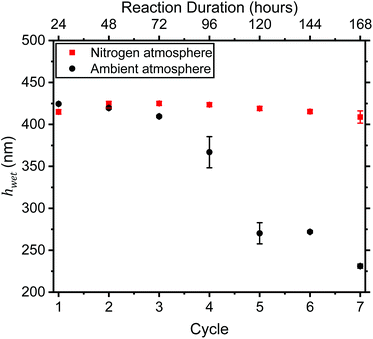
To our knowledge, there are few literature examples of reusing monomer solution for repeated and/or continuous surface-initiated polymerisations cycles (see, for example Zhang et al.,24). After a surface-initiated polymerisation has been carried out, the remaining solution is usually disposed of immediately, regardless of its potential for use in another reaction and if any monomer remains. Since most surface-initiated polymerisations are carried out with a vast excess of monomer, it is likely that monomer remains. The potential for recycling solution and copper wire catalyst was assessed (Fig. 5). Each polymerization cycle was run for 24 hours, after which the functionalised wafer was removed, replaced with a new initiator-coated sample and left for another 24-hour period, for a total of 7 cycles. The results show clearly that monomer remains after for the duration of the experiment with a wet thickness of ∼425 nm being grown for 7 consecutive cycles over a total of 168 hours in a nitrogen atmosphere. It is likely that this process could continue for more cycles, with little drop-off in grown thickness being seen. This result is beneficial for scale-up and high-throughput industrial application, drastically reducing waste amounts and increasing process efficiency compared to individual polymerisations. Furthermore, this result may pave the way for continuous processing (e.g. roll-to-roll).For solution left in ambient conditions and not degassed, a clear drop in grown thickness is observed after the third cycle. By this time, as reported above, the viscosity of the solution had increased significantly. It is likely that either monomer had been consumed in a side Fenton reaction, and/or the resultant high viscosity has significantly affected reagent diffusion and so polymerization kinetics.
4. Conclusions
We have successfully functionalised silicon wafers with thick PMETAC brushes of water-swollen 425 nm thickness, via a more environmentally benign and sustainable Cu(0)-mediated radical polymerisation route that requires no added copper halide catalyst. We detail a system with a cheap and readily-available copper wire catalyst, which can easily be reused. The method was also carried out in tap water, extending previous research by Mendonça et al.,8 to surface-initiated polymer brushes. Further, we have reused the solution over multiple cycles, significantly increasing the number of samples that can be prepared from the same amount of polymerisation solution, making this method of polymerisation highly material- and time-efficient. We demonstrate that our polymerizations work well in ambient conditions, drastically reducing the times involved with deoxygenating solution and the glassware required for polymerisation. However, it is important to note that a side Fenton-type is observed in the presence of oxygen, reducing the method's effectiveness over multiple cycles when in contact with oxygen. It is vital that our system is translated across to other monomer systems in order to help minimise the contribution of polymer chemistry to the environmental crisis and aid the growth of a true bio-economy that is currently strived for.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Department of Physics and Astronomy at the University of Manchester for use of the ellipsometer. They also acknowledge Alastair Bewsher for his help analysing ion chromatography samples. The authors acknowledge funding from the EPSRC (award reference: 1745307).Notes and references
- O. Rzhepishevska, S. Hakobyan, R. Ruhal, J. Gautrot, D. Barbero and M. Ramstedt, Biomater. Sci., 2013, 1, 589 RSC.
- Y. J. Oh, E. S. Khan, A. del Campo, P. Hinterdorfer and B. Li, ACS Appl. Mater. Interfaces, 2019, 11, 29312–29319 CrossRef CAS PubMed.
- M. K. Singh, P. Ilg, R. M. Espinosa-marzal, N. D. Spencer and M. Kröger, Polymers, 2016, 8, 254–261 CrossRef PubMed.
- X. Huang and C. B. Roth, ACS Macro Lett., 2018, 7, 269–274 CrossRef CAS.
- S. Varma and L. Bureau, Langmuir, 2016, 16, 3152–3163 CrossRef PubMed.
- H. Murata, R. R. Koepsel, K. Matyjaszewski and A. J. Russell, Biomaterials, 2007, 28, 4870–4879 CrossRef CAS PubMed.
- J. Huang, R. R. Koepsel, H. Murata, W. Wu, S. B. Lee, T. Kowalewski, A. J. Russell and K. Matyjaszewski, Langmuir, 2008, 24, 6785–6795 CrossRef CAS PubMed.
- P. V. Mendonça, A. S. R. Oliveira, J. P. M. Ribeiro, A. Castilho, A. C. Serra and J. F. J. Coelho, Polym. Chem., 2019, 10, 938–944 Search PubMed.
- P. Anastas and N. Eghbali, Green Chemistry: Principles and Practice, 2010, vol. 39 Search PubMed.
- N. Politakos, S. Azinas and S. E. Moya, Macromol. Rapid Commun., 2016, 37, 662–667 CAS.
- S. G. Boyes, A. M. Granville, M. Baum, B. Akgun, B. K. Mirous and W. J. Brittain, Surf. Sci., 2004, 570, 1–12 CrossRef CAS.
- T. Zhou, H. Qi, L. Han, D. Barbash and C. Y. Li, Nat. Commun., 2016, 7, 11119 CrossRef CAS PubMed.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- C. Barner-Kowollik and S. Perrier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5715–5723 CrossRef CAS.
- X. Pan, M. Fantin, F. Yuan and K. Matyjaszewski, Chem. Soc. Rev., 2018, 47, 5457–5490 RSC.
- K. Matyjaszewski, H. Dong, W. Jakubowski, J. Pietrasik and A. Kusumo, Langmuir, 2007, 23, 4528–4531 CrossRef CAS PubMed.
- S. Wang, J. Song, Y. Li, X. Zhao, L. Chen, G. Li, L. Wang, Z. Jia and X. Ge, React. Funct. Polym., 2019, 140, 48–55 CrossRef CAS.
- R. Ramirez, J. Woodcock and S. M. Kilbey, Soft Matter, 2018, 14, 6290–6302 RSC.
- G. Panzarasa, G. Soliveri and S. Ardizzone, Colloids Surf., A, 2016, 506, 833–839 CrossRef CAS.
- A. J. D. Magenau, Y. Kwak and K. Matyjaszewski, Macromolecules, 2010, 43, 9682–9689 CrossRef CAS.
- F. Alsubaie, A. Anastasaki, V. Nikolaou, A. Simula, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Macromolecules, 2015, 48, 6421–6432 CrossRef CAS.
- E. Liarou, R. Whitfield, A. Anastasaki, N. G. Engelis, G. R. Jones, K. Velonia and D. M. Haddleton, Angew. Chem., Int. Ed., 2018, 57, 8998–9002 CrossRef CAS PubMed.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker and D. M. Haddleton, Chem. Rev., 2016, 116, 835–877 CrossRef CAS PubMed.
- T. Zhang, Y. Du, F. Müller, I. Amin and R. Jordan, Polym. Chem., 2015, 6, 2726–2733 RSC.
- T. Zhang, Y. Du, J. Kalbacova, R. Schubel, R. D. Rodriguez, T. Chen, D. R. T. Zahn and R. Jordan, Polym. Chem., 2015, 6, 8176–8183 RSC.
- S. Edmondson, N. T. Nguyen, A. L. Lewis and S. P. Armes, Langmuir, 2010, 26, 7216–7226 CrossRef CAS PubMed.
- F. Zhang, K. Sautter, A. M. Larsen, D. A. Findley, R. C. Davis, H. Samha and M. R. Linford, Langmuir, 2010, 26, 14648–14654 CrossRef CAS PubMed.
- A. R. Yadav, R. Sriram, J. A. Carter and B. L. Miller, Mater. Sci. Eng., C, 2014, 35, 283–290 CrossRef CAS PubMed.
- E. T. Vandenberg, L. Bertilsson, B. Liedberg, K. Uvdal, R. Erlandsson, H. Elwing and I. Lundström, J. Colloid Interface Sci., 1991, 147, 103–118 CrossRef CAS.
- C. M. Herzinger, B. Johs, W. A. McGahan, J. A. Woollam and W. Paulson, J. Appl. Phys., 1998, 83, 3323–3336 CrossRef CAS.
- E. Palik, Handbook of optical constants of solids, 1985 Search PubMed.
- H. G. Ni, H. Zeng, S. Tao and E. Y. Zeng, Environ. Toxicol. Chem., 2010, 29, 1237–1247 CAS.
- R. Verma, K. S. Vinoda, M. Papireddy and A. N. S. Gowda, Procedia Environ. Sci., 2016, 35, 701–708 CrossRef CAS.
- Y. Kwak, A. J. D. Magenau and K. Matyjaszewski, Macromolecules, 2011, 44, 811–819 CrossRef CAS.
- S. B. Lee, R. R. Koepsel, S. W. Morley, K. Matyjaszewski, Y. Sun and A. J. Russell, Biomacromolecules, 2004, 5, 877–882 CrossRef CAS PubMed.
- W. J. Brittain and S. Minko, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3505–3512 CrossRef CAS.
- M. Biesalski, J. Rühe and J. Ru, Macromolecules, 2002, 35, 499–507 CrossRef CAS.
- B. Zuo, S. Zhang, C. Niu, H. Zhou, S. Sun and X. Wang, Soft Matter, 2017, 13, 2426–2436 RSC.
- N. Cheng, O. Azzaroni, S. Moya and W. T. S. Huck, Macromol. Rapid Commun., 2006, 27, 1632–1636 CrossRef CAS.
- A. Simakova, S. E. Averick, D. Konkolewicz and K. Matyjaszewski, Macromolecules, 2012, 45, 6371–6379 CrossRef CAS.
- J. Yeow, R. Chapman, J. Xu and C. Boyer, Polym. Chem., 2017, 8, 5012–5022 RSC.
- R. Chapman, A. J. Gormley, K.-L. Herpoldt and M. M. Stevens, Macromolecules, 2014, 47, 8541–8547 CrossRef CAS.
- F. Qie, A. Astolfo, M. Wickramaratna, M. Behe, M. D. M. Evans, T. C. Hughes, X. Hao and T. Tan, Nanoscale, 2015, 7, 2480–2488 RSC.
- A. Reyhani, T. G. McKenzie, H. Ranji-Burachaloo, Q. Fu and G. G. Qiao, Chem. – Eur. J., 2017, 23, 7221–7226 CrossRef CAS PubMed.
- J. Yeow, R. Chapman, A. J. Gormley and C. Boyer, Chem. Soc. Rev., 2018, 47, 4357–4387 RSC.
- A. E. Enciso, L. Fu, A. J. Russell and K. Matyjaszewski, Angew. Chem., Int. Ed., 2018, 57, 933–936 CrossRef CAS PubMed.
- A. I. B. Romo, V. S. Dibo, D. S. Abreu, M. S. P. Carepo, A. C. Neira, I. Castillo, L. Lemus, O. R. Nascimento, P. V. Bernhardt, E. H. S. Sousa and I. C. N. Diógenes, Dalton Trans., 2019, 48, 14128–14137 RSC.
- P. Zhou, J. Zhang, Y. Zhang, Y. Liu, J. Liang, B. Liu and W. Zhang, RSC Adv., 2016, 6, 38541–38547 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Ellipsometric model for analysis of brushes in solvent; representative AFM images of PMETAC brush samples; images of change in viscosity of METAC solution; 1H NMR spectra of PMETAC solution without initiator strips. See DOI: 10.1039/d0py00516a |
| This journal is © The Royal Society of Chemistry 2020 |