Synthesis and characterization of light-degradable bromocoumarin functionalized polycarbonates†
Abstract
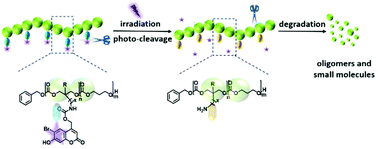
Biocompatible polymeric materials that can degrade in response to external irradiation with high spatial and temporal control show enormous potential in the field of biomedical applications. Herein, two six-membered cyclic carbonate monomers with a light-cleavable bromocoumarin functional pendent moiety attached via a carbamate linkage were prepared from 2-amino-1,3-propanediol (serinol) and 2-(aminomethyl)-2-methylpropane-1,3-diol, respectively. A series of light-degradable polycarbonates were synthesized by organo-catalyzed ring opening polymerization (ROP) of both monomers, and meanwhile their polymerization kinetics were investigated. Upon light-induced deprotection, both types of polycarbonates degraded rapidly into low molecular-weight compounds. The results of size exclusion chromatography (SEC), nuclear magnetic resonance (NMR) spectroscopy and ultraviolet/visible (UV/VIS) spectroscopy confirmed the polymer structures and the light-induced degradation behavior. The photo-cleavage rates of bromocoumarin pendent groups and the degradation rates of the polymers were strongly dependent on polymer structures and used solvents.


 Please wait while we load your content...
Please wait while we load your content...