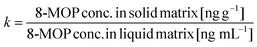Open Access Article
Open Access ArticleMatrix-dependent absorption of 8-methoxypsoralen in extracorporeal photopheresis
Viola
Hähnel
 a,
Isabell
Weber
b,
Simon
Tuemmler
b,
Bernhard
Graf
a,
Isabell
Weber
b,
Simon
Tuemmler
b,
Bernhard
Graf
 b,
Michael
Gruber
b,
Michael
Gruber
 *b,
Ralph
Burkhardt
*b,
Ralph
Burkhardt
 a and
Norbert
Ahrens
a and
Norbert
Ahrens
 a
a
aInstitute of Clinical Chemistry and Laboratory Medicine, Transfusion Medicine, University Hospital Regensburg, Regensburg, Germany
bDepartment of Anesthesiology, University Hospital Regensburg, Regensburg, Germany. E-mail: michael.gruber@ukr.de; Tel: +49 (0) 941/944-7870
First published on 8th July 2020
Abstract
Background: Extracorporeal photopheresis (ECP) is an effective immunomodulatory therapy for various diseases. Autologous leukocytes are collected, photoactivated with a photosensitizer (8-methoxypsoralen, 8-MOP) and UVA light, and subsequently reinfused back to the patient. Leukapheresis and UVA irradiation systems can be integrated into one device (inline) or handled by two separate devices (offline). ECP works via intercalation of 8-MOP into DNA helices and UVA-based interactions to inhibit DNA replication. 8-MOP is known to adhere to plastic materials, which might reduce its availability for intercalation. In the present study we examined the bioavailability of 8-MOP when different plastic materials and solvents are used as matrices. Methods: Varying amounts of shredded ethylene vinyl acetate (EVA) and polyvinylchloride (PVC) from the MacoGenic irradiation bag (EVA1), UVA PIT irradiation bag (EVA2), UVA PIT recirculation bag (PVC A) and UVA PIT tubing (PVC B) by MacoPharma and PIT Medical Systems, respectively, were incubated with 200 ng mL−1 8-MOP dissolved in diisopropyl ether (DIPE) plus toluene 90/10 vol%, deionized water or plasma. After 2 h 8-MOP concentrations were determined by GC-MS. Results: After incubation, 8-MOP concentrations varied depending on the amount and type of plastic (PVC > EVA) and solvent (water > plasma > DIPE/toluene). Absorption to 200 mg EVA1 or EVA2 resulted in 8-MOP concentrations of 57% or 32% in water, 91% or 80% in plasma, and 93% or 92% in DIPE/toluene, while 200 mg PVC A and PVC B yielded recovery rates of 26% and 10% in water, 76% and 75% in plasma, and 55% and 30% in DIPE/toluene, respectively. Remaining 8-MOP differed significantly between container materials (EVA vs. PVC; p < 0.022) but not manufacturers (MacoPharma vs. PIT Medical Systems). Conclusion: Absorption loss of 8-MOP depends on the type of plastic and solvent and is more pronounced with water than with plasma. As the DNA binding effect of 8-MOP is dose-dependent, ECP starting doses should be adjusted to ensure that a sufficient concentration of free bioavailable 8-MOP is present during UV irradiation.
Introduction
Extracorporeal photopheresis (ECP) is a well-established therapy for various immunological diseases such as cutaneous T-cell lymphoma, graft-versus-host-disease or solid organ transplant rejection.1–3 In this three-stage procedure, autologous leukocytes are collected (leukapheresis), incubated ex vivo with photoactive 8-methoxypsoralen (8-MOP) and subsequently exposed to UVA (photoactivation) before being returned to the patient (reinfusion). Leukocyte apheresis and photoactivation may be integrated into a single system (inline) or performed on two separate systems (offline). In offline ECP, 8-MOP-treated leukocytes are transferred in an irradiation bag to a second device for UVA treatment. After photoactivation, the cells are reinfused back to the patient.8-MOP intercalates into DNA and, following exposure to UVA, forms monoadducts and DNA interstrand cross-links,4–7 resulting in the inhibition of DNA replication, white blood cell apoptosis and, ultimately, immunosuppression.8,9 After administration, the unbound fraction of 8-MOP is rapidly metabolized and excreted in the urine.10
8-MOP binds to DNA of cells, to protein and membrane structures and may absorb to plastic materials due to its lipophilic structure.11 The resulting loss of the drug could be dependent on the type of plastic container and solvent (liquid matrix) in which the treated cells are suspended (e.g., plasma or physiological saline). As the binding of 8-MOP to DNA is dose-dependent,12 the starting dose would need to be adjusted to ensure that sufficient quantities of 8-MOP are present in the cell suspension during reinfusion.
Several methods have been described for psoralen detection, including gas chromatography (GC), liquid chromatography-mass spectrometry (LC-MS), and surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI-TOF MS).13–18 In this study, we applied a GC-MS method to examine the adsorption-dependent drug bioavailability of 8-MOP utilizing various commonly used plastic materials and solvents in the liquid and solid matrix.
Materials and methods
Experimental setup
Solid matrices consisted of ECP container materials from two manufacturers: MacoPharma (MacoGenic G2 system) and PIT Medical Systems (UVA PIT system). Ethylene vinyl acetate (EVA) and polyvinylchloride (PVC) sample material was collected from the MacoGenic irradiation bag (EVA 1), UVA PIT irradiation bag (EVA 2), UVA PIT recirculation bag (PVC A) and UVA PIT tubing (PVC B) and manually shredded.Absorption was tested with zero, 80, 100, 120, 150, 180 and 200 mg of sample material (5–6 each) that was incubated for 2 hours with 200 ng 8-MOP from stock solution dissolved in 1000 μL of liquid matrix—diisopropyl ether (DIPE; Honeywell) plus toluene 90/10 v/v, deionized water or plasma (BRK Munich), respectively. 8-MOP stock solutions (2000 ng mL−1) were prepared by toluene extraction of 8-MOP (Uvadex, Therakos, Pennsylvania, USA) in the case of DIPE/toluene, and by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution of 8-MOP in deionized water in the case of plasma and water.
10 dilution of 8-MOP in deionized water in the case of plasma and water.
Aliquots of 200 μL supernatant were transferred to glass tubes. We used 5-MOP (Sigma Aldrich, Germany) as internal standard in a concentration of 20.8 ng μL−1. In triplicate experiments, the psoralens were extracted from deionized water or plasma with toluene (200 μL). The combined toluene fractions were then vaporized at 40 °C and subsequently re-dissolved in 300 μL toluene before analysis. With the DIPE/toluene matrix, the samples were dried in a gentle stream of N2 at 40 °C and directly re-dissolved in 300 μL toluene. A blank sample containing 200 μL solvent alone was always co-tested.
For recovery from absorption (elution), the plastic samples were surface-cleaned with 1000 μL toluene after the above mentioned incubation, transferred to separate tubes and subsequently incubated in 1 mL toluene for 24 hours. 290 μL supernatant and 10 μL internal standard were mixed and analyzed by GC-MS.
The solid to liquid partition coefficients (k) were estimated by the following equation to avoid density inaccuracies:
Gas chromatography
8-MOP concentrations in solvent supernatants were quantified by GC-MS using a 6890 Series Gas Chromatograph with a 5973 Mass Selective Detector (Agilent). Chromatographic separation was performed on a Zebron ZB-5MSPLUS capillary GC column (30 m × 0.25 mm × 0.25 μm; Phenomenex Inc., Torrance, USA). Injection (0.3 μL) was accomplished with the Zebron GC inlet liner in splitless mode. The injector conditions were as follows: 0.965 bar (vent pressure), 280 °C (initial temperature), 1.0 min (purge time), and 2.0 mL min−1 (purge flow). The total flow rate was 6.1 mL min−1. The GC temperature was programmed as follows: 70 °C for 1 min at a heat rate of 10 K min−1 to 320 °C at a carrier gas flow rate of 1.5 mL min−1. The transfer line temperature was 310 °C and the carrier gas was helium. The mass spectrometer was used in EI SIM mode with a solvent delay of 10.5 min, and a cycle time of 14.29 cycles per second (retention times and m/z: 8-MOP 16.8 min; 216; 5-MOP 17.0 min, 173). Regulation and evaluation was achieved using MSD ChemStation software (Agilent Technologies, Santa Clara, USA).Statistical analysis
Microsoft Excel 2010 and IBM SPSS Statistics were used to determine median, mean and standard deviation values, to test for normal distribution using the Kolmogorov–Smirnov test, and to create the figures. Initial median concentrations of free drug in solvent (not adhered to plastic materials) were defined as 100%. Kruskal–Wallis p-values below 0.05 were considered significant.Results
Absorption of 8-MOP varies depending on the type of liquid matrix
With water as the liquid matrix, remaining 8-MOP in the solvent decreased with plastic sample weight from 24% (80 mg plastic) to 10% (200 mg) for PVC A, from 48% (80 mg) to 26% (200 mg) for PVC B (Fig. 1a), from 77% (80 mg) to 57% (200 mg) for EVA 1, and from 47% (80 mg) to 32% (200 mg) for EVA 2.With DIPE/toluene (organic solution) as the liquid matrix, remaining 8-MOP decreased with sample weight from 62% (80 mg) to 30% (200 mg) for PVC A and from 72% (80 mg) to 55% (200 mg) for PVC B (Fig. 1b) compared to decreases from 97% (80 mg) to 92% (200 mg) for EVA2 and 93% (200 mg) for EVA 1.
Compared to the other liquid matrices, plasma resulted in less loss of 8-MOP with remaining 72% (PVC A) and 76% (PVC B, Fig. 1c), and 80% (EVA1) and 91% (EVA2, all 200 mg each).
Absorption of 8-MOP varies depending on the type of solid matrix
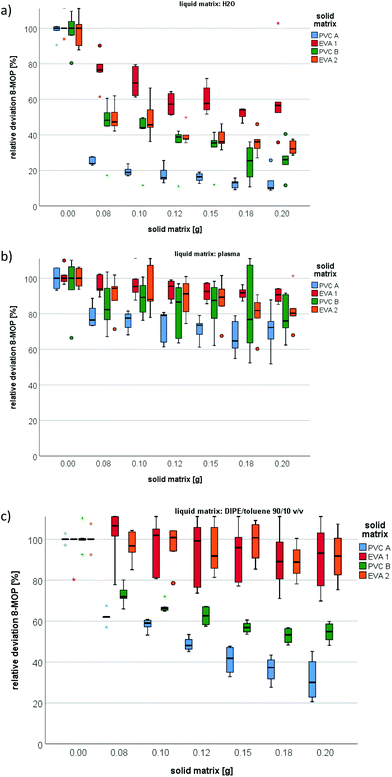
With 200 mg EVA from Macopharma (EVA1) and PIT (EVA2) as the solid matrix, residual 8-MOP concentrations were 57% and 32% in water (Fig. 1a), 91% and 80% in plasma (Fig. 1b), and 93% and 92% in DIPE/toluene, respectively (Fig. 1c). In contrast, even as little as 80 mg of PVC A or PVC B decreased the residual 8-MOP concentration, especially in water (24% and 48%, Fig. 1a) and DIPE/toluene (62% and 72%, Fig. 1c), respectively. The mass of plastic material correlated with the absolute amount of absorption loss of 8-MOP to PVC and, to a lesser extent, to EVA. The PVC materials had similar absorption profiles characterized by a continuous increase in 8-MOP loss in solutions with increasing plastic mass (Fig. 1). The different plastic materials showed significant differences in all three liquid matrices (Table 1).| (a) P value | (b) P value | |
|---|---|---|
| a: EVA 1 (MacoGenic irradiation bag) versus PVC A (UVA PIT recirculation bag); b: EVA 1 (MacoGenic irradiation bag) versus EVA 2 (UVA PIT irradiation bag); n.s., p ≥ 0.05. | ||
| Deionized water | <0.01 | n.s. |
| DIPE/toluene | <0.01 | 0.014 |
| Plasma | 0.022 | n.s. |
Recovery of absorbed 8-MOP
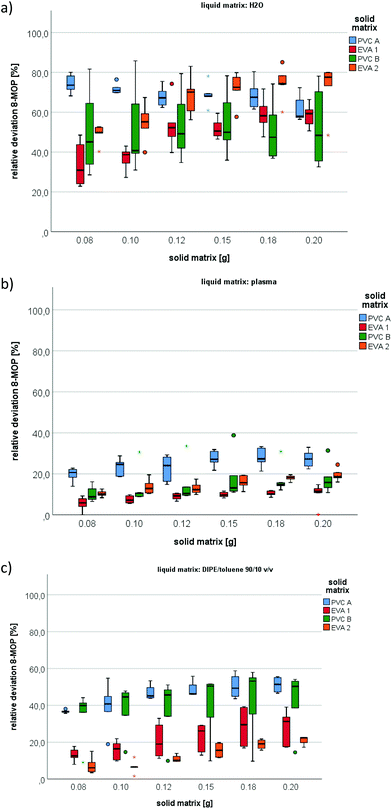
To verify that 8-MOP loss was due to absorption into the plastic samples and not into the glass incubation tubes or other factors, we additionally eluted 8-MOP from the plastic samples. The results confirmed that 8-MOP loss was due to absorption by the respective solid matrix (Fig. 2). Of 200 mg 8-MOP that was absorbed to PVC A, 51%, 58%, and 27% could be retrieved with DIPE/toluene, water, and plasma, respectively. The corresponding rates for 200 mg PVC B were 50% (DIPE/toluene), 48% (water) and 16% (plasma). These results concur with those of previous experiments and confirm the loss of 8-MOP due to absorption.Samples of EVA1 and EVA2 absorbed less of the photochemical, as reflected by recovery rates of 34% and 22% (DIPE/toluene), 59% and 78% (water) and 11% and 19% (plasma), respectively.
In general, the higher affinity of 8-MOP to PVC, especially in water, is illustrated by the solid to liquid partition coefficients (Fig. 3). Partition coefficients for EVA were 7.7, 0.8, and 1.2 in water, plasma, and DIPE/toluene, while those for PVC were 29.2, 2.3, and 5.5, respectively.
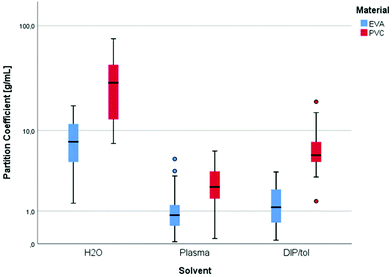 | ||
| Fig. 3 Partition coefficients of 8-MOP [g mL−1] dependent on the type of plastic material and solvent. | ||
Discussion
ECP is an established immunomodulatory therapy for diverse diseases.3 The patient's leukocytes are treated with the photoactive substance 8-MOP and irradiated with UVA. This induces immunological effects, such as the apoptosis of lymphocytes and the induction of regulatory T-cells. This effect is dependent on different factors that influence the uptake of 8-MOP into the cells. It includes parameters like the 8-MOP concentration, the irradiation dose or the amount of time the cell suspension is incubated with 8-MOP before irradiation.19This study was conducted to simulate the influence of different solvents and container materials on the bioavailability of 8-MOP during ECP. Irradiation bags usually consist of EVA, a plastic material which should have less affinity to 8-MOP than others. Nevertheless, about 30% of free bioavailable 8-MOP is lost due to adsorption to EVA.20
Loss of 8-MOP from the liquid matrix into the solid matrix varied dependent on the type and surface area of plastic material in the solid matrix as well as on the type of solvent in the liquid matrix (Table 2). Our test results indicated that 8-MOP was more likely absorbed into the plastic matrix rather than just being adsorbed onto its surface. PVC absorbed more 8-MOP than EVA. Incubation with increasing amounts of plastic material resulted in decreasing concentrations of free 8-MOP in solvent (recovery). Absorption loss was more pronounced in water than in plasma.
| Deionized water | Plasma | DIPE/toluene | |
|---|---|---|---|
| Symbols: weak (↑), moderate (↑↑), strong (↑↑↑), unchanged (↔). | |||
| EVA | ↑↑ | ↔ | ↔ |
| PVC | ↑↑↑ | ↑ | ↑↑ |
Similarly, Laulhé et al. observed that 8-MOP adsorption was significantly higher in NaCl (79%) than in plasma (94%), resulting in the inhibition of lymphocyte proliferation and an increase in T-cell apoptosis.21 Hence, plasma seems to be associated with lower bioavailability of 8-MOP even though adsorption is less pronounced. This could be caused by protein binding of 8-MOP in plasma.
In conclusion, the type of liquid matrix in which leukocytes are suspended influences the availability of 8-MOP. To ensure that free, bioavailable drug is present during ECP treatment, the starting dose should be considered accordingly.
Conflicts of interest
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Photochemical and Photobiological Sciences.References
- J. Schwartz, A. Padmanabhan, N. Aqui, R. A. Balogun, L. Connelly-Smith, M. Delaney, N. M. Dunbar, V. Witt, Y. Wu and B. H. Shaz, J. Clin. Apher., 2016, 31, 149–162 Search PubMed
.
- J. Adamski, T. Kinard, T. Ipe and L. Cooling, Transfus. Apher. Sci., 2015, 52, 171–182 CrossRef PubMed
.
- A. Alfred, P. C. Taylor, F. Dignan, K. El-Ghariani, J. Griffin, A. R. Gennery, D. Bonney, E. Das-Gupta, S. Lawson, R. K. Malladi, K. W. Douglas, T. Maher, J. Guest, L. Hartlett, A. J. Fisher, F. Child and J. J. Scarisbrick, Br. J. Haematol., 2017, 177, 287–310 CrossRef PubMed
.
- P. S. Song and K. J. Tapley Jr., Photochem. Photobiol., 1979, 29, 1177–1197 CrossRef CAS PubMed
.
- H. Cao, J. E. Hearst, L. Corash and Y. Wang, Anal. Chem., 2008, 80, 2932–2938 CrossRef CAS PubMed
.
- G. D. Cimino, H. B. Gamper, S. T. Isaacs and J. E. Hearst, Annu. Rev. Biochem., 1985, 54, 1151–1193 CrossRef CAS PubMed
.
- T. Bessho, J. Biol. Chem., 2003, 278, 5250–5254 CrossRef CAS
.
- D. Schmid, C. Grabmer, D. Streif, T. Lener, K. Schallmoser and E. Rohde, Vox Sang., 2015, 108, 82–88 CrossRef CAS PubMed
.
- C. Franklin, E. Cesko, U. Hillen, B. Schilling and S. Brandau, PLoS One, 2015, 10, e0134518 CrossRef PubMed
.
- Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG), Mol. Nutr. Food Res., 2007, 51, 367–373 CrossRef PubMed
.
- S. E. Shephard, F. O. Nestle and R. Panizzon, Photodermatol., Photoimmunol. Photomed., 1999, 15, 64–74 CrossRef CAS PubMed
.
- J. Kruger, E. Christophers and M. Schlaak, Br. J. Dermatol., 1978, 98, 141–144 CrossRef CAS PubMed
.
- S. I. Smith and J. S. Brodbelt, Analyst, 2010, 135, 943–952 RSC
.
- J. Gazith, W. Schalla and H. Schaefer, Arch. Dermatol. Res., 1978, 263, 215–222 CrossRef CAS PubMed
.
- J. Malakova, P. Zak, I. Jokesova, P. Zivny, M. Blazek, A. Zavrelova and V. Palicka, Chromatographia, 2009, 69, 779–783 CrossRef CAS
.
- H. Ehrsson, S. Eksborg, I. Wallin, N. Kallberg and G. Swanbeck, J. Chromatogr., 1977, 140, 157–164 CrossRef CAS PubMed
.
- A. D. Buhimschi and F. P. Gasparro, Photochem. Photobiol., 2014, 90, 241–246 CrossRef CAS PubMed
.
- J. Li, Q. H. Zhang, J. He, E. W. Liu, X. M. Gao and Y. X. Chang, Sci. World J., 2015, 2015, 365093 Search PubMed
.
- L. Karolak, M. Tod, A. Leon, A. M. Heudes, O. Petitjean and L. Laroche, Photodermatol., Photoimmunol. Photomed., 1992, 9, 58–60 CAS
.
- V. Hähnel, F. Dormann, A. Nitsopoulos, A. Friedle and N. Ahrens, Photochem. Photobiol. Sci., 2017, 16, 193–200 RSC
.
- M. Laulhe, S. Lefebvre, D. Le Broc-Ryckewaert, M. Pierre, A. Ferry and B. Delorme, PLoS One, 2019, 14, e0212835 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |