 Open Access Article
Open Access ArticleUltraviolet-B radiation exposure lowers the antioxidant capacity in the Arabidopsis thaliana pdx1.3-1 mutant and leads to glucosinolate biosynthesis alteration in both wild type and mutant†
Susanne
Neugart
a,
Éva
Hideg
 b,
Gyula
Czégény
b,
Gyula
Czégény
 b,
Monika
Schreiner
c and
Åke
Strid
b,
Monika
Schreiner
c and
Åke
Strid
 *d
*d
aDivision of Quality and Sensory of Plant Products, University of Göttingen, Göttingen, Germany
bDepartment of Plant Biology, University of Pécs, Pécs, Hungary
cDepartment of Plant Quality and Food Security, Leibniz Institute of Vegetable and Ornamental Crops, Großbeeren, Germany
dSchool of Science & Technology, Örebro Life Science Center, Örebro University, Örebro, Sweden. E-mail: ake.strid@oru.se
First published on 21st January 2020
Abstract
Pyridoxine (vitamin B6) and its vitamers are used by living organisms both as enzymatic cofactors and as antioxidants. We used Arabidopsis pyridoxine biosynthesis mutant pdx1.3-1 to study the involvement of the PLP-synthase main polypeptide PDX1 in plant responses to ultraviolet radiation of two different qualities, one containing primarily UV-A (315–400 nm) and the other containing both UV-A and UV-B (280–315 nm). The antioxidant capacity and the flavonoid and glucosinolate (GS) profiles were examined. As an indicator of stress, Fv/Fm of photosystem II reaction centers was used. In pdx1.3-1, UV-A + B exposure led to a significant 5% decrease in Fv/Fm on the last day (day 15), indicating mild stress at this time point. The antioxidant capacity of Col-0 wildtype increased significantly (50–73%) after 1 and 3 days of UV-A + B. Instead, in pdx1.3-1, the antioxidant capacity significantly decreased by 44–52% over the same time period, proving the importance of a full complement of functional PDX1 genes for the detoxification of reactive oxygen species. There were no significant changes in the flavonoid glycoside profile under any light condition. However, the GS profile was significantly altered, both with respect to Arabidopsis accession and exposure to UV. The difference in flavonoid and GS profiles reflects that the GS biosynthesis pathway contains at least one pyridoxine-dependent enzyme, whereas no such enzyme is used in flavonoid biosynthesis. Also, there was strong correlation between the antioxidant capacity and the content of some GS compounds. Our results show that vitamin B6 vitamers, functioning both as antioxidants and co-factors, are of importance for the physiological fitness of plants.
Introduction
Vitamin B6 (VitB6) is an essential cofactor involved in over 140 different biochemical reactions.1 In living cells there are primarily six different VitB6 analogues, the so called vitamers: pyridoxine (PN), pyridoxal (PL), pyridoxamine (PM) and their respective phosphorylated versions (PNP, PLP, and PMP, respectively). Of the phosphorylated VitB6 species, PLP (pyridoxal 5′-phosphate) is the most important, since it is the vitamer that is active as a catalytic cofactor in anabolic and catabolic enzymes. In an in silico study, Percudani and Peracchi2 found that the classes of enzymes that most commonly used PLP as a cofactor were transferases, lyases, isomerases, hydrolases, and oxidoreductases, in descending order of appearance in enzymes that were anticipated to carry this cofactor. In the model plant Arabidopsis thaliana, more than 100 genes were assigned to be encoding PLP-binding proteins.2–5 Mutant studies revealed that decreased VitB6 synthesis capabilities led to developmental and morphological changes. This included reduced shoot, root, and leaf development,6–10 and delayed flowering time.8In plants, PLP is synthesized de novo from glutamine, ribose 5-phosphate, and glyceraldehyde 3-phosphate by two interacting proteins, the PDX1 PLP synthase and the PDX2 glutaminase.1,4,11 PDX2 extracts ammonium from glutamine and delivers it to PDX1 which synthesizes the final product. PDX1 is a multifunctional enzyme catalysing at least six different chemical reactions. It has been suggested to be one of the most complicated enzymes that exists,1 with regard to the intricate catalytic processes that are carried out by the polypeptide. The PDX1 PLP synthase exists as a dodecamer in vivo1 and the details of its catalytic function are at present being unveiled.1,12
With regard to the physiological role of VitB6, increasing scientific evidence shows that VitB6 also can act as an antioxidant, in addition to its role as a cofactor.6,13 VitB6 efficiently detoxifies various reactive oxygen species (ROS) such as singlet oxygen, superoxide anion radical, hydroxyl radical, and hydrogen peroxide.6,13–19 Thus, in plants, the relatively newly discovered antioxidant activity of VitB6 has increased the interest of understanding VitB6 function under different types of environmental stresses.5 This includes salt stress,6,7 osmotic stress,6 photoinhibition,7,20 and biotic stress and disease resistance.21,22 Furthermore, VitB6 has been shown to play an important role in plant responses to ultraviolet-B radiation (UV-B; 280–315 nm), which is a naturally occurring part of the solar spectrum. This role is manifested as an increased expression of VitB6 biosynthesis genes and enzymes21,22 and the accumulation of VitB6 in plants exposed to UV-B.22 In fact, it is possible that during plant stress in general, VitB6 could either act as an antioxidant, or as an important cofactor in the stress-protective metabolism, or both. In the A. thaliana VitB6 biosynthesis mutant pdx1.3-1, first characterized by Titiz et al.,7 we have previously shown that H2O2 accumulated to a larger extent than in the Col-0 wild type and that the photosynthetic parameters Fv/Fm (maximal dark-adapted PSII quantum yield) and Y(II) (effective light-acclimated PSII quantum yield) decreased, whereas the non-photochemical quenching due to dissipative processes (Y(NO)) increased.18 Again, this indicated that the pdx1.3-1 mutant, lacking full capacity for VitB6 synthesis, suffered from UV-B-induced oxidative stress. It should be noted that of the three PDX1 genes, only PDX1.1 and PDX1.3 are functional and the PDX1.3 dominates in the wild type plant.7 The pdx1.1pdx1.3 double mutant is embryo lethal.7
In order to further examine the role of VitB6 under conditions of supplementary UV-B radiation at levels that generally are not stressful for plants, we devised the present study. Here we further examined the antioxidant capacity of wild type and pdx1.3-1 plants exposed to supplementary UV-A radiation or UV-A + B radiation, and in plants exposed to photosynthetically active radiation (PAR) only. In addition, we analysed the content and profile of two classes of phytochemicals, flavonoids and glucosinolates (GS). Flavonoids were chosen because their synthesis is a signature UV response in plants23 and since their biosynthesis is independent of enzymes using VitB6 as the cofactor. In the case of GS24–26 they were chosen since one step in the core GS biosynthesis has been confirmed to be dependent on VitB6, i.e. the one catalysed by the SUR1 S-alkyl-thiohydroximate lyase.27 Also, three more enzymes involved in the amino acid interconversion leading up to GS biosynthesis have been inferred to use PLP as the cofactor: the branched-chain-amino-acid aminotransferases 3 (BCAT3;25http://www.uniprot.org/uniprot/Q9M401), 4 (BCAT4;28http://www.uniprot.org/uniprot/Q9LE06), and 6 (BCAT6;29http://www.uniprot.org/uniprot/Q9LPM9), respectively. Thus, with respect to the glucosinolate metabolism, the effects of both the exposure to supplementary UV radiation and the VitB6 deficiency in the core glucosinolate biosynthesis could be detected.
Materials and methods
Materials, growth of plants, light and UV-B exposure conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) and the PDX1.3 T-DNA insertion mutant line pdx1.3-1 (SALK_086418) were obtained from The European Arabidopsis Stock Centre, Nottingham, UK (http://arabidopsis.org). Seeds were sown in an Arasystem Arabidopsis growth system (Betatech bvba, Ghent, Belgium) on a fertilized compost![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) perlite (75
perlite (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) mixture and incubated in darkness at 4 °C, for 3–5 days. The plants were thereafter grown at 150–190 μmol photons m−2 s−1 of PAR using a 16 h light/8 h darkness photoperiod at 22 °C. 40 days after sowing the plants were in addition to the PAR irradiated with UV-A + UV-B (0.25 W m−2) or UV-A only (0.11 W m−2). The UV-B source (Philips TL40 W/12UV) was covered either with cellulose acetate (UV-A + UV-B) or with Mylar film (UV-A only) to exclude wavelengths shorter than approximately 292 and 315 nm, respectively (Fig. 1A). The cellulose acetate and Mylar filters have minimal influence on the visible light exposure of the plants. The plant-weighted UV-B radiation30,31 was 36.1 mW m−2 for the UV-A + B exposed plants and 0.03 mW m−2 for the UV-A exposed plants (Fig. 1B), corresponding to daily doses of 781 and 1 J m−2, respectively. The UV irradiance was measured using an OL754 spectroradiometer (Optronic Laboratories Inc., Orlando, FL). Plants were UV exposed each day for 6 h centered around the solar noon for 1–15 days. Attached leaves were used for chlorophyll fluorescence. All leaves used for antioxidant and flavonoid and GS phytochemical analysis were harvested after cessation of UV exposure on each indicated day and frozen in liquid nitrogen and kept at −80 °C until freeze drying.
25) mixture and incubated in darkness at 4 °C, for 3–5 days. The plants were thereafter grown at 150–190 μmol photons m−2 s−1 of PAR using a 16 h light/8 h darkness photoperiod at 22 °C. 40 days after sowing the plants were in addition to the PAR irradiated with UV-A + UV-B (0.25 W m−2) or UV-A only (0.11 W m−2). The UV-B source (Philips TL40 W/12UV) was covered either with cellulose acetate (UV-A + UV-B) or with Mylar film (UV-A only) to exclude wavelengths shorter than approximately 292 and 315 nm, respectively (Fig. 1A). The cellulose acetate and Mylar filters have minimal influence on the visible light exposure of the plants. The plant-weighted UV-B radiation30,31 was 36.1 mW m−2 for the UV-A + B exposed plants and 0.03 mW m−2 for the UV-A exposed plants (Fig. 1B), corresponding to daily doses of 781 and 1 J m−2, respectively. The UV irradiance was measured using an OL754 spectroradiometer (Optronic Laboratories Inc., Orlando, FL). Plants were UV exposed each day for 6 h centered around the solar noon for 1–15 days. Attached leaves were used for chlorophyll fluorescence. All leaves used for antioxidant and flavonoid and GS phytochemical analysis were harvested after cessation of UV exposure on each indicated day and frozen in liquid nitrogen and kept at −80 °C until freeze drying.
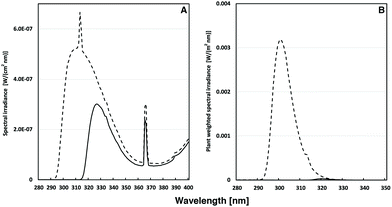 | ||
| Fig. 1 (A) Spectral UV irradiance (in W (cm2 nm)−1) of Philips TL40/12 UV tubes used for the irradiation of A. thaliana plants. The UV-A + B light regime (dashed line) was accomplished by filtering the light through cellulose acetate sheets. For the UV-A-enriched light (solid line), Mylar sheets were used as filters. In (B) the corresponding plant-weighted UV (in W (m2 nm)−1)30 during the two types of exposures is shown. | ||
Chlorophyll fluorescence measurement
The maximal efficiency of PSII (Fv/Fm)32 was measured using a Plant Efficiency Analyzer (PEA; Hansatech Instruments, United Kingdom). After 15 min of dark adaptation a minimum of three plants per treatment and at least two of the youngest fully developed leaves from each plant were measured, making the total of measured leaves, n = 6–17. One measurement per leaf was performed. The means and standard deviations of these measurements were calculated.Antioxidant capacity assay
Hydroxyl radical neutralizing capacities were determined according to Šnyrychová and Hideg.33 The method is based on the ability of antioxidants contained in leaf extracts to decrease the oxidation of terephthalate (TPA) to hydroxy-terephthalate (HTPA) by hydroxyl radicals generated in the assay. This antioxidant capacity was characterized by the amount of plant sample needed to decrease HTPA fluorescence (315 nm excitation, 420 nm emission) by 50%, as described earlier.34 The reaction mixture contained 500 μM terephthalate, 100 μM ascorbate, 10 μM Fe(II)SO4, and 10 μM EDTA in 50 mM Na-phosphate buffer (pH 7.0). The reaction was started by adding 100 μM H2O2. The assay was calibrated with ethanol, which has high reactivity to hydroxyl radicals, and data were given as mM ethanol equivalent per g leaf dry weight.Flavonoid and hydroxycinnamic acid analysis
Flavonoid glycosides and hydroxycinnamic acid derivatives were analyzed according to Schmidt et al.35 with slight modification. Lyophilized, ground plant material (0.02 g) was extracted with 600 μl of 60% aqueous methanol on a magnetic stirrer plate for 40 min at 20 °C. The extract was centrifuged at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at the same temperature, and the supernatant was collected in a reaction tube. This process was repeated twice with 300 μl of 60% aqueous methanol for 20 and 10 min, respectively; the three corresponding supernatants were combined. The extract was subsequently evaporated until it was dry and was then suspended in 200 μl of 10% aqueous methanol. The extract was centrifuged at 14
000g for 10 min at the same temperature, and the supernatant was collected in a reaction tube. This process was repeated twice with 300 μl of 60% aqueous methanol for 20 and 10 min, respectively; the three corresponding supernatants were combined. The extract was subsequently evaporated until it was dry and was then suspended in 200 μl of 10% aqueous methanol. The extract was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g for 5 min at 20 °C through a Corning Costar Spin-X plastic centrifuge tube filter (Sigma Aldrich Chemical Co., St Louis, MO) for HPLC analysis. Each extraction was carried out in duplicate.
500g for 5 min at 20 °C through a Corning Costar Spin-X plastic centrifuge tube filter (Sigma Aldrich Chemical Co., St Louis, MO) for HPLC analysis. Each extraction was carried out in duplicate.
The composition and content of flavonoid glycosides and hydroxycinnamic acid derivatives were determined from the filtrate using a series 1100 HPLC (Agilent Technologies, Waldbronn, Germany) equipped with a degasser, binary pump, autosampler, column oven, and photodiode array detector. An Ascentis Express F5 column (150 mm × 4.6 mm, 5 μm, Supelco) was used to separate the compounds at 25 °C. Eluent A was 0.5% acetic acid, and eluent B was 100% acetonitrile. The gradient used for eluent B was 5–12% (0–3 min), 12–25% (3–46 min), 25–90% (46–49.5 min), 90% isocratic (49.5–52 min), 90–5% (52–52.7 min), and 5% isocratic (52.7–59 min). The determination was conducted at a flow rate of 0.85 ml min−1 and a wavelength of 280 nm, 320 nm, 330 nm, 370 nm, and 520 nm. The hydroxycinnamic acid derivatives and glycosides of flavonoids were tentatively identified as deprotonated molecular ions and characteristic mass fragment ions according to Schmidt et al.35 and Neugart et al.36 by HPLC-DAD-ESI-MSn using an Bruker amazon SL ion trap mass spectrometer in negative ionisation mode. For the identification of the peaks, the data were compared with the literature of the investigated species and their derivatives. In the mass spectrometer, nitrogen was used as the dry gas (10 L min−1, 325 °C) and the nebulizer gas (40 psi) with a capillary voltage of −3500 V. Helium was used as the collision gas in the ion trap. The mass optimization for the ion optics of the mass spectrometer for quercetin was performed at m/z 301 or arbitrarily at m/z 1000. The MSn experiments were performed in auto up to MS3 in a scan from m/z 200–2000. Standards (chlorogenic acid, quercetin 3-glucoside, kaempferol 3-glucoside and isorhamnetin-3-glucoside; Roth, Karlsruhe, Germany) were used for external calibration curves in a semi-quantitative approach. Results are presented as mg per g dry weight.
Glucosinolate analysis
The glucosinolate composition of the samples was determined as desulfo-glucosinolates, using a slightly modified method according to Wiesner et al.37 The modifications were as follows: the various desulfo-glucosinolates were separated on a UHPLC-DAD device (UHPLC Agilent 1290 Infinity System, Agilent Technologies, Böblingen, Germany) equipped with a Poroshell 120 EC-C18 column of dimension 100 mm × 2.1 mm containing particles of size 2.7 μm (Agilent Technologies). The solvent gradient was formed using water (A) and 40% acetonitrile (B), starting at 0.5% B for 2 min, increasing to 49.5% B over the next 10 min, then held for a further 2 min, increased to 99.5% B over the course of 1 min and then held for a final 2 min. The flow rate was 0.4 mL min−1 and the injection volume was 5 μL. Desulfo-glucosinolates were identified by comparing retention times and UV absorption spectra with those of known standards. Quantification was done at 229 nm using the response factor of the GS relative to 2-propenyl glucosinolate (external standard). The determination of glucosinolates was performed in duplicate.Statistical analysis
MS Excel was used to calculate the means and standard deviations. Pairwise differences between means were evaluated using two sample Student t-tests. The Wizard software (version 1.9.29) for Macintosh was used for this purpose. The number of sampling elements (N) in each sample is indicated in corresponding figures. Probabilities of the null hypothesis that the two means are equal (p values) are indicated in figures as * p < 0.05; ** p < 0.01; *** p < 0.005; and **** p < 0.001. The comparison of treatments (ANOVA) for each phenolic compound was done using Tukey's HSD test at a significance level of 5%.Correlations between the amounts of glucosinolate compounds and hydroxyl radical neutralizing antioxidant capacities were analysed by calculating Person's correlation coefficient (r). Probabilities of the null hypothesis that there is no correlation (p-values) are shown together with r-values. A linear connection between hydroxyl radical neutralizing antioxidant capacities and amounts of one of the glucosinolate compounds was studied by calculating a linear fit using the method of least squares. Following this, the probability of the null hypothesis that the slope of a fitting line is equal to zero was examined. The coefficient of determination (R2) was also calculated. These analyses were carried out using PAST software38 and Statistica™ for Windows™ (version 13.0, Statsoft Inc., Tulsa, OK. USA).
Results and discussion
While phenolic compounds such as flavonoids and phenolic acids are well known antioxidants in UV-B defense, VitB6 recently gained attention in the process of cellular oxidative stress.39 VitB6 has high antioxidant capacity against hydroxyl radicals17 and other ROS. Concomitantly, A. thaliana pdx1.3-1 mutants are highly sensitive to UV-B.18,20 It has also been shown that the VitB6 content increased after UV-B but not UV-A exposure in the A. thaliana Col-0 wildtype but not in pdx1.3-1.22 However, it has been inferred in a theoretical study that phenolic compounds may be stronger antioxidants than VitB6.40 Indeed, increased flavonoid content in plant leaves due to UV-B exposure is a well-known plant response,41 whereas the increase in PDX protein levels is comparably small.41 On the other hand, UV-A radiation did not have any major effect on the flavonoid abundance of Arabidopsis leaves.41Therefore, we devised the present experimental set-up to study the role of VitB6 vitamers in the UV-B-regulation of flavonoid and GS profiles on the one hand, and the antioxidant capacity of leaves of the Col-0 and pdx1.3-1 A. thaliana accessions, on the other. As a proxy for distress,42 we also measured the dark-adapted maximal photosynthetic capacity in the form of the chlorophyll fluorescence parameter Fv/Fm. This parameter has previously been shown to differ between the two Arabidopsis varieties after a short, high dose UV exposure.18 Thus, the use of low dose exposure as in the present study is novel.
Maximum quantum yield of PSII photochemistry, Fv/Fm
F v/Fm was measured in A. thaliana plants exposed to control light (PAR only), UV-A- or UV-A + B-supplemented PAR. The exposures were carried out for 0, 1, 2, 3, 4, 8, or 15 days, six hours per day centered around the solar noon. No significant differences were found as a function of genotype per se. Before day 15, significant decreases in Fv/Fm compared with the corresponding controls were only found on day 3 and 4 for the pdx1.3-1 mutant illuminated with supplementary UV-A + B (Fig. 2; Table 1). This may reflect insufficient protection due to decreased antioxidant capacity in pdx1.3-1 leaves (see below).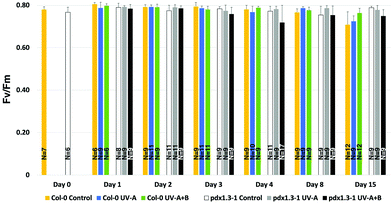 | ||
| Fig. 2 Maximum quantum yield of PSII photochemistry (Fv/Fm) was measured in A. thaliana plants exposed to control light, UV-A- or UV-A + B-supplemented light, given for 0, 1, 2, 3, 4, 8, or 15 days, six hours per day centered around the solar noon. The bars indicate the standard deviation. Significant changes between pairwise physiologically relevant comparisons of samples are shown in Table 1 and means and standard deviations in Table S1.†N = 6–17 leaves from at least three different plants were measured. | ||
| Day | Genotype | Treatment | Significance | Day | Genotype | Treatment |
|---|---|---|---|---|---|---|
| 0 | Col-0 | Control | *** | 1 | Col-0 | Control |
| 0 | Col-0 | Control | ** | 15 | Col-0 | Control |
| 3 | pdx1.3-1 | Control | * | 3 | pdx1.3-1 | UV-A + B |
| 4 | Col-0 | UV-A | * | 4 | Col-0 | UV-A + B |
| 4 | pdx1.3-1 | Control | * | 4 | pdx1.3-1 | UV-A + B |
| 4 | pdx1.3-1 | UV-A | * | 4 | pdx1.3-1 | UV-A + B |
| 8 | Col-0 | Control | * | 8 | Col-0 | UV-A |
| 8 | Col-0 | Control | * | 15 | Col-0 | Control |
| 8 | Col-0 | UV-A | **** | 15 | Col-0 | UV-A |
| 8 | pdx1.3-1 | Control | * | 8 | pdx1.3-1 | UV-A |
| 8 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | Control |
| 8 | pdx1.3-1 | UV-A | * | 8 | pdx1.3-1 | UV-A + B |
| 15 | Col-0 | Control | ** | 15 | Col-0 | UV-A + B |
| 15 | Col-0 | Control | **** | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | UV-A | *** | 15 | Col-0 | UV-A + B |
| 15 | pdx1.3-1 | Control | **** | 15 | pdx1.3-1 | UV-A + B |
| 15 | pdx1.3-1 | UV-A | * | 15 | pdx1.3-1 | UV-A + B |
Interestingly, changes in Fv/Fm were seen also after 15 days, although this was not the case for the 8-day exposures. In fact, there was a small but significant difference in Fv/Fm in Col-0 on day 15 between the control and UV-A-treated plants on the one hand, and the UV-A + B-treated plants on the other (by 0.039–0.055 units). Also, there was a significantly lower Fv/Fm in the Col-0 control plants than in the pdx1.3-1 controls after 15 days of exposure (by 0.080 units). Lowering of the Fv/Fm in Col-0 on day 15 may be attributed to the first signs of bolting that had occurred in all nine plants in this accession at that time-point but only in one of the nine pdx1.3-1 plants (not shown). Thus, the pdx1.3-1 mutation may lead to delayed bolting compared with the wild type. This may in turn be due to a lower abundance in pdx1.3-1 of metabolites needed for the transition from the vegetative to the reproductive stage.
However, this finding is in contrast to the case in the A. thaliana pdx3 mutant.43 The pdx3 mutant is deficient in the gene encoding the PDX3 PMP/PNP oxidase of the VitB6 salvage pathway that functions to interconvert the vitamers PMP and PNP to PLP. The pdx3 mutant instead displayed an early flowering phenotype that was linked to the over-accumulation of PMP and a concomitant impaired nitrogen metabolism.43 In addition, bolting in itself may considerably alter the metabolism of Arabidopsis plants, e.g. in the form of increased oxidative pressure as a result of decreased ascorbate peroxidase and catalase activities.44,45
Taken together, these results indicate that the plants had not been subjected to any distress42 caused by the UV treatments. However, eustress may have occurred in the pdx1.3-1 plants exposed to UV-A + B, particularly after 3 days of exposure or longer.
The hydroxyl radical neutralizing capacity
The antioxidant capacity of mature (40-day old) Col-0 wild type and pdx1.3-1 mutant plants was measured. This was done 3 hours after the solar noon on the day before the onset of UV-A or UV-A + B exposure (day 0), or at 3 hours after the solar noon on the first and third day of UV exposure (day 1 and day 3, respectively). As is evident from Fig. 3 and Table 2, UV-A exposure did not give rise to any changes in this antioxidant capacity, neither after 1 nor 3 days of exposure, in any of the two Arabidopsis accessions, compared with the controls. However, there was a strongly significant increase in antioxidant capacity in Col-0 already after exposure to supplementary UV-A + B radiation for one day. An equally significant decrease in antioxidant capacity in pdx1.3-1 indicates the lack of induction of any protective response against the highly oxidizing hydroxyl radical in UV-B exposed plants when only one functional PDX1 gene was present.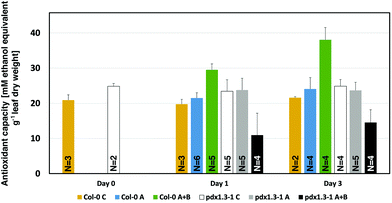 | ||
| Fig. 3 Hydroxyl radical antioxidant capacity measurement in A. thaliana Col-0 wildtype and pdx1.3-1 mutant plants 0, 1, and 3 days (6 hours per day around the solar noon) after supplementary UV-A or UV-A + B exposure. The bars indicate the standard deviation. Significant changes between pairwise physiologically relevant comparisons of samples are shown in Table 2. N = 2–6 replicates per sample type. | ||
| Day | Genotype | Treatment | Significance | Day | Genotype | Treatment |
|---|---|---|---|---|---|---|
| 0 | Col-0 | Control | * | 0 | pdx1.3-1 | control |
| 1 | Col-0 | Control | **** | 1 | Col-0 | UV-A + B |
| 1 | Col-0 | Control | * | 1 | pdx1.3-1 | control |
| 1 | Col-0 | UV-A | **** | 1 | Col-0 | UV-A + B |
| 1 | Col-0 | UV-A + B | *** | 3 | Col-0 | UV-A + B |
| 1 | Col-0 | UV-A + B | **** | 1 | pdx1.3-1 | UV-A + B |
| 1 | pdx1.3-1 | Control | ** | 1 | pdx1.3-1 | UV-A + B |
| 1 | pdx1.3-1 | UV-A | ** | 1 | pdx1.3-1 | UV-A + B |
| 3 | Col-0 | Control | *** | 3 | Col-0 | UV-A + B |
| 3 | Col-0 | UV-A | **** | 3 | Col-0 | UV-A + B |
| 3 | Col-0 | UV-A + B | **** | 3 | pdx1.3-1 | UV-A + B |
| 3 | pdx1.3-1 | Control | *** | 3 | pdx1.3-1 | UV-A + B |
| 3 | pdx1.3-1 | UV-A | *** | 3 | pdx1.3-1 | UV-A + B |
Interestingly, in the pdx1.3-1 mutant the ˙OH neutralizing antioxidant capacity of the control plants (day 0, day 1, and day 3; Fig. 3), is higher than in the corresponding controls by 15–19%. This is similar to the case in another PDX1 Arabidopsis mutant (rsr4-1) used in UV-B exposure studies.19 The reason for this higher background antioxidant capacity in rsr4-1 was attributed to the contribution of ˙OH scavenging by other non-enzymatic antioxidants. Particularly chlorogenic acid (a plant phenolic compound) and α-tocopherol were considered the main candidates for this.19
Flavonoids and sinapic acid
The analysis of the flavonoid glycosides and hydroxycinnamic acid derivatives in leaves of Arabidopsis Col-0 and the pdx1.3-1 mutant revealed a highly dynamic picture with regard to the total content and the relative abundance of each of the chemical species over 15 days of UV exposure (Tables 3 and 4). In general, sinapic acid and its derivatives were more prominent constituents in younger than in older leaves. The total levels of flavonoids remained constant over time.| Days of exposure | Treatment | Genotype | Q-3-rha-7-glc | K-3-rha-7-rha | K-3-glc-7-rha | K-3-rut-7-glc | Average: total flavonoids | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Control | Col-0 | 5.10 ± 3.84 | 7.49 ± 0.97 | 1.94 ± 2.73 | 5.24 ± 0.19 | B | 22.98 ± 2.89 | B | ||
| UV-A | Col-0 | 1.79 ± 1.16 | 5.07 ± 0.13 | 1.84 ± 1.03 | 2.85 ± 0.06 | AB | 15.31 ± 1.45 | AB | |||
| UV-A + B | Col-0 | 0.53 ± 0.43 | 4.06 ± 3.14 | 1.97 ± 1.62 | 2.25 ± 1.73 | A | 9.69 ± 7.23 | A | |||
| Control | pdx1.3-1 | 3.51 ± 5.01 | 8.50 ± 0.35 | B | 5.18 ± 1.37 | B | 6.34 ± 0.61 | B | 31.20 ± 6.77 | B | |
| UV-A | pdx1.3-1 | 0.62 ± 0.24 | 4.73 ± 0.50 | A | 2.66 ± 0.47 | A | 2.45 ± 0.15 | A | 14.16 ± 1.08 | A | |
| UV-A + B | pdx1.3-1 | 0.89 ± 0.13 | 4.93 ± 1.60 | A | 1.98 ± 0.82 | A | 2.58 ± 0.60 | A | 12.45 ± 2.17 | A | |
| 4 | Control | Col-0 | 0.15 ± 0.02 | 8.54 ± 0.83 | AB | 1.26 ± 0.19 | 4.82 ± 0.22 | 18.85 ± 2.43 | AB | ||
| UV-A | Col-0 | 0.19 ± 0.05 | 5.69 ± 2.90 | A | 0.94 ± 0.51 | 3.25 ± 1.87 | 13.01 ± 6.33 | A | |||
| UV-A + B | Col-0 | 0.28 ± 0.12 | 11.56 ± 0.69 | B | 1.28 ± 1.15 | 7.22 ± 3.32 | 25.55 ± 4.73 | B | |||
| Control | pdx1.3-1 | 0.49 ± 0.43 | 4.96 ± 1.00 | A | 1.67 ± 0.82 | A | 2.90 ± 0.13 | A | 12.04 ± 2.23 | A | |
| UV-A | pdx1.3-1 | 0.53 ± 0.57 | 4.64 ± 1.49 | A | 0.97 ± 0.72 | A | 2.68 ± 0.63 | A | 11.54 ± 2.69 | A | |
| UV-A + B | pdx1.3-1 | 0.35 ± 0.30 | 10.49 ± 0.72 | B | 5.02 ± 1.08 | B | 7.35 ± 0.23 | B | 27.58 ± 1.14 | B | |
| 8 | Control | Col-0 | 0.13 ± 0.01 | 3.71 ± 1.16 | 2.05 ± 0.47 | 2.25 ± 0.64 | 8.56 ± 2.19 | A | |||
| UV-A | Col-0 | 0.33 ± 0.26 | 4.69 ± 2.15 | 1.30 ± 0.44 | 2.61 ± 1.24 | 10.00 ± 3.956 | A | ||||
| UV-A + B | Col-0 | 0.12 ± 0.01 | 8.42 ± 2.81 | 4.43 ± 3.38 | 6.56 ± 2.83 | 21.34 ± 4.11 | B | ||||
| Control | pdx1.3-1 | 0.13 ± 0.01 | 2.94 ± 0.52 | 1.40 ± 0.33 | 1.66 ± 0.29 | 6.51 ± 1.13 | A | ||||
| UV-A | pdx1.3-1 | 0.32 ± 0.04 | 3.42 ± 1.73 | 2.89 ± 1.13 | 1.98 ± 0.93 | 10.60 ± 1.02 | A | ||||
| UV-A + B | pdx1.3-1 | 0.43 ± 0.29 | 7.50 ± 4.87 | 1.33 ± 1.36 | 9.47 ± 2.46 | 22.31 ± 3.17 | B | ||||
| 15 | Control | Col-0 | 0.29 ± 0.03 | 4.53 ± 0.83 | A | 1.93 ± 0.42 | 2.71 ± 0.48 | A | 11.25 ± 1.71 | A | |
| UV-A | Col-0 | 0.34 ± 0.27 | 4.69 ± 1.30 | A | 2.12 ± 0.63 | 2.57 ± 1.75 | A | 10.94 ± 3.68 | A | ||
| UV-A + B | Col-0 | 0.24 ± 0.11 | 10.99 ± 0.48 | B | 6.15 ± 4.96 | 8.61 ± 2.93 | B | 26.44 ± 8.43 | B | ||
| Control | pdx1.3-1 | 0.48 ± 0.31 | 3.81 ± 0.51 | A | 0.90 ± 0.46 | 2.26 ± 0.15 | A | 8.30 ± 0.70 | A | ||
| UV-A | pdx1.3-1 | 0.44 ± 0.22 | 4.54 ± 0.68 | A | 1.70 ± 0.18 | 2.66 ± 0.54 | A | 10.78 ± 0.61 | A | ||
| UV-A + B | pdx1.3-1 | 0.29 ± 0.07 | 10.72 ± 0.83 | B | 3.64 ± 4.57 | 9.77 ± 0.73 | B | 24.96 ± 6.02 | B | ||
| Days of exposure | Treatment | Genotype | Sinapoyl-glucoside | Sinapoyl-malate | Sinapic acid | Average: total sinapic acid derivatives | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Control | Col-0 | 0.11 ± 0.06 | a | 0.99 ± 0.75 | ab | 0.51 ± 0.04 | b | 1.61 ± 0.72 | b | ||||
| UV-A | Col-0 | 0.18 ± 0.03 | 0.74 ± 0.34 | 0.96 ± 0.91 | 1.88 ± 0.64 | |||||||||
| UV-A + B | Col-0 | 0.12 ± 0.10 | 0.10 ± 0.03 | 0.30 ± 0.20 | 0.52 ± 0.31 | |||||||||
| Control | pdx1.3-1 | 0.12 ± 0.05 | 2.69 ± 1.63 | B | 1.03 ± 1.53 | 3.83 ± 1.39 | B | |||||||
| UV-A | pdx1.3-1 | 0.24 ± 0.25 | 0.43 ± 0.09 | AB | 1.18 ± 0.93 | 1.85 ± 0.61 | AB | |||||||
| UV-A + B | pdx1.3-1 | 0.08 ± 0.00 | 0.17 ± 0.07 | A | 0.78 ± 0.43 | 1.03 ± 0.50 | A | |||||||
| 4 | Control | Col-0 | 0.88 ± 0.48 | b | 0.93 ± 0.14 | A | b | 0.23 ± 0.04 | a | 2.04 ± 0.66 | b | |||
| UV-A | Col-0 | 0.46 ± 0.47 | 0.60 ± 0.56 | A | 0.41 ± 0.48 | 1.47 ± 0.84 | ||||||||
| UV-A + B | Col-0 | 0.32 ± 0.31 | 2.20 ± 0.27 | B | 0.08 ± 0.01 | 2.60 ± 0.12 | ||||||||
| Control | pdx1.3-1 | 0.37 ± 0.48 | 0.50 ± 0.23 | 0.14 ± 0.10 | 1.01 ± 0.81 | |||||||||
| UV-A | pdx1.3-1 | 0.19 ± 0.19 | 0.60 ± 0.70 | 0.57 ± 0.46 | 1.35 ± 0.36 | |||||||||
| UV-A + B | pdx1.3-1 | 0.73 ± 0.57 | 1.34 ± 1.11 | 0.11 ± 0.05 | 2.18 ± 0.82 | |||||||||
| 8 | Control | Col-0 | 0.06 ± 0.00 | a | 0.06 ± 0.00 | a | 0.09 ± 0.01 | a | 0.21 ± 0.01 | a | ||||
| UV-A | Col-0 | 0.11 ± 0.07 | 0.14 ± 0.03 | 0.29 ± 0.29 | 0.54 ± 0.35 | |||||||||
| UV-A + B | Col-0 | 0.07 ± 0.05 | 0.77 ± 0.57 | 0.07 ± 0.00 | 0.90 ± 0.56 | |||||||||
| Control | pdx1.3-1 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.19 ± 0.00 | |||||||||
| UV-A | pdx1.3-1 | 0.06 ± 0.00 | 0.29 ± 0.12 | 0.64 ± 0.90 | 0.99 ± 0.94 | |||||||||
| UV-A + B | pdx1.3-1 | 0.08 ± 0.03 | 1.07 ± 1.06 | 0.64 ± 0.43 | 1.79 ± 1.23 | |||||||||
| 15 | Control | Col-0 | 0.34 ± 0.24 | a | 0.21 ± 0.23 | a | 0.35 ± 0.29 | a | 0.90 ± 0.26 | B | a | |||
| UV-A | Col-0 | 0.24 ± 0.16 | 0.09 ± 0.01 | 0.29 ± 0.03 | 0.61 ± 0.16 | AB | ||||||||
| UV-A + B | Col-0 | 0.07 ± 0.00 | 0.09 ± 0.04 | 0.06 ± 0.00 | 0.22 ± 0.04 | A | ||||||||
| Control | pdx1.3-1 | 0.06 ± 0.00 | A | 0.22 ± 0.13 | 0.14 ± 0.06 | AB | 0.42 ± 0.07 | |||||||
| UV-A | pdx1.3-1 | 0.06 ± 0.00 | A | 0.17 ± 0.05 | 0.49 ± 0.28 | B | 0.72 ± 0.32 | |||||||
| UV-A + B | pdx1.3-1 | 0.07 ± 0.00 | B | 0.13 ± 0.03 | 0.07 ± 0.01 | A | 0.27 ± 0.03 | |||||||
The major sinapic acid derivative sinapoyl-malate increased after 4 days of UV-A + B exposure in Col-0 whereas sinapoyl glucoside increased after 15 days of UV-A + B exposure in pdx1.3-1 (Table 4). Thus, our data indicate that sinapic acid derivatives do not contribute to any large extent in Arabidopsis UV defense. In fact, as shown by Heinze et al.,46 these compounds decrease in abundance with plant maturation and only partly vary with UV irradiation during growth. Although an increased content of hydroxycinnamic acid derivatives has been suggested to be an important response to UV exposure in A. thaliana,47 we have generally found these compounds to be less important than flavonoid glycosides.48
For flavonoid glycosides, there was a general shift in total flavonoid content between day 2 and the later days of the study. In the 2-day time point, the total flavonoid levels in both Arabidopsis accessions were higher in the non-UV controls. In the later time-points the UV-A + B-exposed plants always had a higher content of flavonoids in leaves than both the controls and the UV-A-exposed plants (Table 3). This is reminiscent of the situation in kale (Brassica oleracea var. sabellica),49 where the quercetin glycoside content decreased after one day of UV-B treatment and increased in the following days of UV-B treatment, concomitantly with the mRNAs encoding flavonol 3′-hydroxylases. The effect of the additional UV-B in the present study was substantial after 4 days and further on.
The difference in the total flavonoid glycoside content and flavonoid glycoside profile between the Col-0 wild type and the pdx1.3-1 mutant was in principle non-existent at all time-points when the same exposure conditions were compared. This underscores the lack of interaction between flavonoid biosynthesis and VitB6.
With regard to the individual flavonoid glycosides, quercetin-3-rhamnoside-7-glucoside was present to a relatively large extent in both Arabidopsis accessions on day 2 in the non-UV control but to a considerably smaller extent after exposure to both UV-based light regimens (Table 3). While Demkura et al.49 and Götz et al.50 found an increase of quercetin glycosides in Arabidopsis after UV-B exposure, our results are again in line with those obtained with kale.51 From day 4 and onwards, the quercetin-glucoside was hardly detectable in any of the samples.
UV-A and UV-A + B exposure led to an initial (day 2) decrease of kaempferol glycosides in pdx1.3-1. UV-A + B exposure then resulted in an increase of these compounds on day 4 in pdx1.3-1 leaves. Kaempferol-3-rhamnoside-7-rhamnoside and kaempferol-3-rutinoside-7-glucoside contents increased in Col-0 and pdx1.3-1 leaves of UV-exposed plants on day 15. Consequently, pdx1.3-1 shows a more extended and faster response to UV. The results support the hypothesis of VitB6 being an antioxidant that can detoxify various ROS.15,17–19 Indeed, the increased content of kaempferol glycosides has previously been shown to be part of the UV response in several Brassicaceae51–53 and in Arabidospsis.49
Glucosinolates
Ten individual glucosinolates were quantitatively analyzed in A. thaliana Col-0 wild type and its mutant pdx1.3.-1. In both genotypes the aliphatic glucosinolates represented the majority (by content and by the number of chemical species) of glucosinolates. Particularly the subgroup of methylsulfinylalkyl glucosinolates (3-methylsulfinylpropyl, 4-methylsulfinylbutyl, 5-methylsulfinylpentyl, 7-methylsulfinyl-heptyl, and 8-methylsulfinylocyl) dominated, primarily in the form of the 4-methylsulfinylbutyl glucosinolate. Precursors of the methylsulfinylalkyl glucosinolates, i.e. methylthioalkyl glucosinolates, were also found, but only as minor components of the total GS. Only two of the five methylsulfinylalkyl glucosinolate precursors were present: 3-methyl-thiopropyl and 4-methylthiobutyl glucosinolates. The group of indole glucosinolates was comprised of the indole 3-indolylmethyl glucosinolate and its derivatives 4-hydroxy-3-indolylmethyl and 1-hydroxy-3-indolylmethyl glucosinolates. However, the latter compounds were only present in trace amounts.The two genotypes could be differentiated by their corresponding individual glucosinolate levels. The pdx1.3-1 mutant (pdx control, in Fig. 4) showed a constitutively higher total glucosinolate content than the Col-0 wild type (col control, in Fig. 4), particularly and significantly with ongoing ontogeny at day 15 (Fig. 4A and Table 5). This significant genotype effect on day 15 was also reflected in the glucosinolate subgroups (aliphatic, methylthioalkyl, methylsulfinylalkyl and indole GS; Fig. 4B–E and Table 5). The higher GS levels in the pdx1.3.-1 mutant were thus present although there was a reduced availability of VitB6 due to the knock-out of one of the two functional PDX1 genes. This may seem surprising since VitB6 is necessary as a cofactor in GS synthesis. PLP is present in BCAT3, BCAT4 and BCAT6 enzymes, and active in the formation of the methionine side-chain elongation as a precursor of aliphatic glucosinolates.29 It is also necessary for the activity of SUR1,27 which is an enzyme synthesizing thiohydroximic acid that in turn is glycosylated and sulfonated to form the core structure of all glucosinolates. However, in our present study, VitB6 is not limiting the glucosinolate formation in pdx1.3-1 and has a higher glucosinolate content than Col-0.
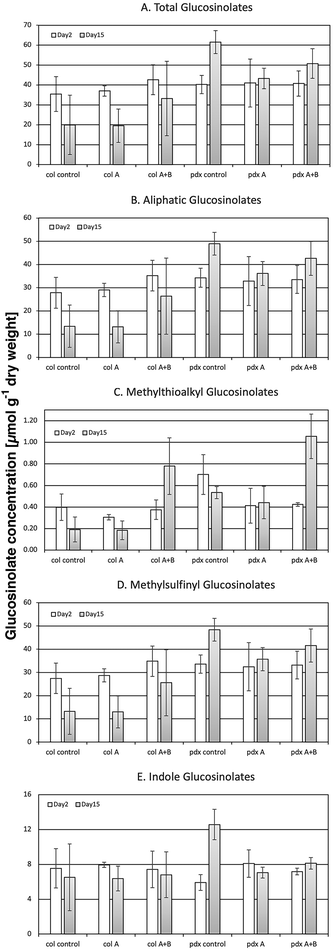 | ||
| Fig. 4 Content (in μmol per g dry weight) of total (A), aliphatic (B), methylthioalkyl (C), methylsulfinylalkyl (D) and indole glucosinolates (E) in Arabidopsis thaliana Col-0 wild type and the pdx1.3-1 mutant exposed to control light, or UV-A- or UV-A + B-supplemented light after 2 and 15 d (n = 3); the bars indicate the standard deviation. Significant changes between pairwise physiologically relevant comparisons of samples are shown in Table 5. | ||
| Day | Genotype | Treatment | Significance | Day | Genotype | Treatment |
|---|---|---|---|---|---|---|
| A | ||||||
| Total GS | ||||||
| 2 | Col-0 | UV-A | * | 15 | Col-0 | UV-A |
| 2 | pdx1.3-1 | Control | ** | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | UV-A | * | 15 | pdx1.3-1 | UV-A |
| 15 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | UV-A |
| B | ||||||
| Aliphatic GS | ||||||
| 2 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | UV-A | ** | 15 | pdx1.3-1 | UV-A |
| 15 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | UV-A |
| C | ||||||
| Methyl thioalkyl GS | ||||||
| 2 | pdx1.3-1 | UV-A + B | ** | 15 | pdx1.3-1 | UV-A + B |
| 15 | Col-0 | Control | * | 15 | Col-0 | UV-A + B |
| 15 | Col-0 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | UV-A | * | 15 | Col-0 | UV-A + B |
| 15 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | UV-A + B |
| 15 | pdx1.3-1 | UV-A | * | 15 | pdx1.3-1 | UV-A + B |
| D | ||||||
| Methyl sulfinyl GS | ||||||
| 2 | Col-0 | UV-A | * | 15 | Col-0 | UV-A |
| 2 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | Control | * | 15 | pdx1.3-1 | Control |
| 15 | Col-0 | UV-A | * | 15 | pdx1.3-1 | UV-A |
| 15 | Col-0 | UV-A + B | * | 15 | pdx1.3-1 | UV-A |
| E | ||||||
| Indole GSs | ||||||
| 2 | pdx1.3-1 | Control | *** | 15 | pdx1.3-1 | Control |
| 15 | pdx1.3-1 | Control | ** | 15 | pdx1.3-1 | UV-A |
| 15 | pdx1.3-1 | Control | * | 15 | pdx1.3-1 | UV-A + B |
In contrast to the case with the flavonoids, the levels of all glucosinolates in the pdx1.3.-1 mutant were significantly reduced under one or both UV treatments, most obvious after extended UV-A treatment on day 15 (Fig. 4B, D, E and Table 5). Although the glucosinolate profile changed differently to UV exposure with respect to plant species,54 Wang et al.55 also reported a decrease in the concentration of total glucosinolates in A. thaliana after 12 h of UV-B treatment (1.55 W m−2). However, in our present study, using considerably lower UV levels (0.7 kJ m−2 d−1 of plant weighted UV-B), nearly all glucosinolates in the Col-0 wild type were unaffected by both UV treatments used (Fig. 4B, D, E and Table 5). Furthermore, Demkura49 showed no effect of UV-B (5.5 kJ m−2 d−1 of plant weighted UV-B) on GS. This lack of UV response indicates that glucosinolates have no primary function in response to UV (especially UV-B) in the way flavonoids have.
Notwithstanding, in both Arabidopsis genotypes, the minor methylthioalkyl glucosinolates were exceptions to the lack of UV response, which is reported here for the first time. On day 15 of exposure, they showed a distinct and statistically significant increase under UV-A + B radiation compared with the corresponding controls and compared with exposures to UV-A only (Fig. 4C and Table 5). This response was primarily determined by the accumulation of the 4-methylthiobutyl glucosinolate, which is a precursor in 4-methylsulfinylbutyl glucosinolate formation. Thus, this effect suggests that under the 15-day UV-A + B exposure, there was a negative impact on the 4-methylthiobutyl to 4-methylsulfinylbutyl side chain oxidation carried out by the flavin monooxgenase FMO GS-OX5 (an enzyme that does not use VitB6 as a co-factor). No such impact was seen after UV-A exposure (Fig. 4C and Table 5). These results were in contrast to a UV study using a shorter UV-A + B exposure at a lower dose.56 In that study, UV-A + B-treated broccoli sprouts (0.3 kJ m−2 d−1 given for 5 days) exhibited an increased methylsulfinylbutyl glucosinolate content. This increase was matched with a corresponding up-regulation of the FMO GS-OX5 gene. Thus, this suggests that there are both UV-related and genetic differences between the two plant species – Arabidopsis vs. broccoli – with regard to the conversion of 4-methylthiobutyl to 4-methylsulfinylbutyl glucosinolate.
Particularly the content of the precursor 4-methylthiobutyl glucosinolate was correlated with a suppression of the antioxidant capacity (Tables 6 & S2†). On the other hand, the content of the more abundant short-chain methylsulfinylalkyl glucosinolates (3-methylsulfinylpropyl, 4-methylsulfinylbutyl) was positively correlated with the hydroxyl radical antioxidant capacity in both Arabidopsis accessions. An increased formation of ROS, e.g. under UV-A + B exposure, can be assumed to result in an increased formation of ROS scavenging metabolites, such as the short-chain methylsulfinylalkyl glucosinolates (Fig. 5), in contrast to the UV-A treatment, which is not expected to lead to ROS formation. However, the decreased conversion of 4-methylthiobutyl to 4-methylsulfinylbutyl glucosinolate prevents the contribution of the latter compound to the antioxidant capacity in both Col-0 and pdx1.3.-1 under UV-A + B treatment. Moreover, in the UV-A-exposed plants there was neither an accumulation of 4-methylthiobutyl glucosinolate nor an increase in 4-methylsulfinylbutyl glucosinolate. This suggests that the antioxidative potential of the short-chain methylsulfinylalkyl glucosinolates is not utilized in these Arabidopsis accessions. Accordingly, Taviano et al.57 proposed that glucosinolates are not directly involved in the primary antioxidant activity. Instead, these authors suggested that the ferrous iron-chelating properties of glucosinolates protect cells from oxidative stress caused by ROS.
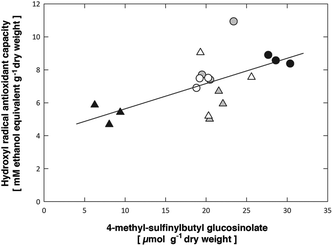 | ||
| Fig. 5 Significant (p = 0.00702) positive correlation between the leaf 4-methyl-sulfinylbutyl glucosinolate content and the hydroxyl radical antioxidant capacities. Symbols show data for Arabidopsis thaliana Col-0 (circles) or pdx1.3-1 mutant leaves (triangles) exposed to control (open), UV-A-supplemented (grey) or UV-A + B-supplemented (black) light. The solid line shows the linear regression, R2 = 0.373 (see Table 6 for the statistical analysis and Table S3† for ordinary least squares regression fit of data). | ||
| Compound | aox-OHrad | 3-m-spr | 4-m-sbut | 5-m-spe | 3-m-tpr | 4-m-tbut | 7-m-shep | i-3-m | 8-m-soct | 4-m-3-im | 1-m-3-im | 7-m-thep | 8-met-toct | Total GS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The following abbreviations were used: aox-OHrad, hydroxyl radical antioxidant capacity; 3-m-spr, 3-methyl-sulfinylpropyl; 4-m-sbut, 4-methyl-sulfinylbutyl; 5-m-spe, 5-methyl-sulfinylpentyl; 3-m-tpr, 3-methyl-thiopropyl; 4-m-tbut, 4-methyl-thiobutyl; 7-m-shep, 7-methyl-sulfinylheptyl; i-3-m, indolyl-3-methyl; 8-m-soct, 8-methyl-sulfinyloctyl; 4-m-3-im, 4-methoxy-3-indolylmethyl; 1-m-3-im, 1-methoxy-3-indolylmethyl; 7-m-thep, 7-methyl-thioheptyl; 8-met-toct total, 8-methyl-thiooctyl; GS, total glucosinolate content. | ||||||||||||||
| aox-OHrad | 0.0160 | 0.0070 | 0.7918 | 0.2482 | 0.0350 | 0.0607 | 0.0333 | 0.9498 | 0.0438 | 0.0977 | 0.3394 | 0.5028 | 0.0126 | |
| 3-m-spr | 0.5583 | 0.0000 | 0.8754 | 0.3779 | 0.0069 | 0.0200 | 0.0000 | 0.6707 | 0.0465 | 0.0106 | 0.5728 | 0.9585 | 0.0000 | |
| 4-m-sbut | 0.6113 | 0.9539 | 0.3373 | 0.0604 | 0.0311 | 0.0008 | 0.0000 | 0.7051 | 0.0047 | 0.0029 | 0.8367 | 0.6801 | 0.0000 | |
| 5-m-spe | 0.0669 | −0.0398 | 0.2401 | 0.0001 | 0.1878 | 0.0028 | 0.5662 | 0.0023 | 0.0066 | 0.3173 | 0.1730 | 0.0894 | 0.1653 | |
| 3-m-tpr | 0.2870 | 0.2211 | 0.4508 | 0.8047 | 0.2728 | 0.0000 | 0.2684 | 0.0001 | 0.0364 | 0.3073 | 0.0751 | 0.0409 | 0.0227 | |
| 4-m-tbut | −0.4990 | −0.6127 | −0.5087 | 0.3253 | 0.2731 | 0.8328 | 0.0270 | 0.0005 | 0.1070 | 0.1375 | 0.0007 | 0.0072 | 0.1065 | |
| 7-m-shep | 0.4504 | 0.5426 | 0.7189 | 0.6610 | 0.8895 | 0.0536 | 0.0104 | 0.0025 | 0.0329 | 0.0749 | 0.1425 | 0.0630 | 0.0001 | |
| i-3-m | 0.5030 | 0.8812 | 0.8899 | 0.1449 | 0.2756 | −0.5199 | 0.5874 | 0.7859 | 0.0172 | 0.0001 | 0.8171 | 0.3982 | 0.0000 | |
| 8-m-soct | 0.0160 | −0.1077 | 0.0959 | 0.6714 | 0.7852 | 0.7334 | 0.6662 | −0.0689 | 0.7952 | 0.9362 | 0.0028 | 0.0043 | 0.4114 | |
| 4-m-3-im | 0.4799 | 0.4747 | 0.6344 | 0.6153 | 0.4959 | −0.3927 | 0.5041 | 0.5532 | 0.0658 | 0.0420 | 0.6343 | 0.8845 | 0.0035 | |
| 1-m-3-im | 0.4026 | 0.5861 | 0.6597 | 0.2499 | 0.2549 | −0.3641 | 0.4300 | 0.7929 | −0.0203 | 0.4837 | 0.6802 | 0.2308 | 0.0012 | |
| 7-m-thep | −0.2390 | −0.1425 | −0.0523 | 0.3359 | 0.4297 | 0.7248 | 0.3598 | 0.0587 | 0.6613 | −0.1204 | 0.1044 | 0.0000 | 0.7192 | |
| 8-met-toct | −0.1689 | −0.0132 | 0.1044 | 0.4119 | 0.4858 | 0.6099 | 0.4469 | 0.2121 | 0.6392 | 0.0369 | 0.2974 | 0.9462 | 0.3251 | |
| Total GS | 0.5747 | 0.9119 | 0.9842 | 0.3416 | 0.5333 | −0.3932 | 0.7880 | 0.9141 | 0.2063 | 0.6497 | 0.7007 | 0.0911 | 0.2460 | |
Conclusions
Our experiments using two different UV light regimens in exposure studies with Col-0 wild type Arabidopsis and A. thaliana plants deficient in VitB6 biosynthesis (pdx1.3-1) led to the following conclusions:1. The use of 15 days of UV-A or UV-A + B exposure with Arabidopsis Col-0 did not lead to any distress, as judged by Fv/Fm chlorophyll fluorescence levels, that were only marginally affected by the exposures. In the pdx1.3-1 mutant, UV-A + B exposure led to a significant 5% decrease in Fv/Fm on day 15 of the experiment, indicating the development of eustress.
2. The antioxidant capacity of Col-0 increased after 1 and 3 days of UV-A + B exposure by at least 50%. In the pdx1.3-1 mutant the antioxidant capacity decreased by at least 40% over the same time period.
3. No significant changes in flavonoid content and profile between Arabidopsis accessions were found. Starting from day 4, UV-A + B treatments led to the highest content of total flavonoid glycosides.
4. The glucosinolate profile was significantly changed, both with regard to the Arabidopsis accession and the UV exposure, during the exposure period. This most likely reflects the presence of at least one VitB6 vitamer cofactor-dependent enzyme in the GS biosynthesis pathway.
5. The antioxidant capacity of the Arabidopsis genotypes and their GS profiles were correlated: short-chain methylsulfinylalkyl GS were positively correlated with the antioxidant capacity in both Col-0 and pdx1.3-1, whereas the content of 4-methylthiobutyl GS was correlated with suppression of the antioxidant capacity.
Our results thus confirm the important and dual role of VitB6 as both an antioxidant and an enzymatic co-factor in plants.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant to Å. S. from the Knowledge Foundation (kks.se; contract no. 20130164), the Swedish Research Council Formas (formas.se/en; contract no. 942-2015-516), and Örebro University's Faculty for Business, Science and Technology. This work was also supported by the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the 20765-3/2018/FEKUTSTRAT ‘Innovation for sustainable and healthy living and environment’ thematic programme of the University of Pécs. Finally, this work was supported by the Research Area Plant Quality and Food Security at Leibniz-Institute of Vegetable and Ornamental Crops.References
- G. C. Robinson, M. Kaufmann, C. Roux and T. B. Fitzpatrick, Structural definition of the lysine swing in Arabidopsis thaliana PDX1: Intermediate channeling facilitating vitamin B6 biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5821–E5829 CrossRef CAS PubMed.
- R. Percudani and A. Peracchi, A genomic overview of pyridoxal-phosphate-dependent enzymes, EMBO Rep., 2003, 4, 850–854 CrossRef CAS PubMed.
- R. Percudani and A. Peracchi, The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families, BMC Bioinf., 2009, 10, 273 CrossRef PubMed.
- S. Mooney and H. Hellmann, Vitamin B6: Killing two birds with one stone?, Phytochemistry, 2010, 71, 495–501 CrossRef CAS PubMed.
- H. Vanderschuren, et al., Strategies for vitamin B6 biofortification of plants: a dual role as a micronutrient and a stress protectant, Front. Plant Sci., 2013, 4, 143 Search PubMed.
- H. Chen and L. Xiong, Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses, Plant J., 2005, 44, 396–408 CrossRef CAS PubMed.
- O. Titiz, et al., PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis, Plant J., 2006, 50, 347–363 Search PubMed.
- S. Wagner, et al., Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of the PDX1 protein family in metabolism, development, and vitamin B6 biosynthesis, Plant Cell, 2006, 18, 1722–1735 CrossRef CAS PubMed.
- M. Raschke, et al., Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis, Plant J., 2011, 66, 414–432 CrossRef CAS PubMed.
- S. Boycheva, A. Dominguez, J. Rolcik, T. Boller and T. B. Fitzpatrick, Consequences of a deficit in vitamin B6 biosynthesis de novo for hormone homeostasis and root development in Arabidopsis, Plant Physiol., 2015, 167, 102–117 CrossRef CAS PubMed.
- M. Tambasco-Studart, et al., Vitamin B6 biosynthesis in higher plants, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13687–13692 CrossRef CAS PubMed.
- M. J. Rodrigues, et al., Lysine relay mechanism coordinates intermediate transfer in vitamin B6 biosynthesis, Nat. Chem. Biol., 2017, 13, 290–294 CrossRef CAS PubMed.
- M. Ehrenshaft, P. Bilski, M. Y. Li, C. F. Chignell and M. E. Daub, A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9374–9378 CrossRef CAS PubMed.
- M. Ristilä, J. M. Matxain, Å. Strid and L. A. Eriksson, pH-dependent electronic and spectroscopic properties of pyridoxine (Vitamin B6), J. Phys. Chem. B, 2006, 110, 16774–16780 CrossRef PubMed.
- J. M. Matxain, M. Ristilä, Å. Strid and L. A. Eriksson, Theoretical study of the antioxidant properties of pyridoxine, J. Phys. Chem. A, 2006, 110, 13068–13072 CrossRef CAS PubMed.
- J. M. Matxain, M. Ristilä, Å. Strid and L. A. Eriksson, Theoretical study of the reaction of vitamin B6 with 1O2, Chem. – Eur. J., 2007, 13, 4636–4642 CrossRef CAS PubMed.
- J. M. Matxain, D. Padro, M. Ristilä, Å. Strid and L. A. Eriksson, Evidence of high ˙OH radical quenching efficiency by vitamin B6, J. Phys. Chem. B, 2009, 113, 9629–9632 CrossRef CAS PubMed.
- Gy. Czégény, et al., Hydrogen peroxide contributes to the ultraviolet-B (280-315 nm) induced oxidative stress of plant leaves through multiple pathways, FEBS Lett., 2014, 588, 2255–2261 CrossRef PubMed.
- Gy. Czégény, et al., Multiple roles for Vitamin B6 in plant acclimation to UV-B, Sci. Rep., 2019, 9, 1259 CrossRef PubMed.
- M. Havaux, et al., Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress, BMC Plant Biol., 2009, 9, 130 CrossRef PubMed.
- M. Brosché, M. A. Schuler, I. Kalbina, L. Connor and Å. Strid, Gene regulation by low level UV-B radiation: identification by DNA array analysis, Photochem. Photobiol. Sci., 2002, 1, 656–664 RSC.
- M. Ristilä, H. Strid, L. A. Eriksson, Å. Strid and H. Sävenstrand, The role of the pyridoxine (vitamin B6) biosynthesis enzyme PDX1 in ultraviolet-B radiation responses in plants, Plant Physiol. Biochem., 2011, 49, 284–292 CrossRef PubMed.
- R. Lois, Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis thaliana L., Planta, 1994, 194, 498–503 CrossRef CAS.
- B. A. Halkier and J. Gershenzon, Biology and biochemistry of glucosinolates, Annu. Rev. Plant Biol., 2006, 57, 303–313 CrossRef CAS PubMed.
- T. Knill, J. Schuster, M. Reichelt, J. Gershenzon and S. Binder, Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis, Plant Physiol., 2008, 146, 1028–1039 CrossRef CAS PubMed.
- A. P. Klein and E. S. Sattely, Biosynthesis of cabbage phytoalexins from indole glucosinolate, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 1910–1915 CrossRef CAS PubMed.
- M. Dalgaard Mikkelsen, P. Naur and B. A. Halkier, Arabidopsis mutants in the C±S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis, Plant J., 2004, 37, 770–777 CrossRef PubMed.
- J. Schuster, T. Knill, M. Reichelt, J. Gershenzon and S. Binder, BRANCHED-CHAIN AMINOTRANSFERASE4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis, Plant Cell, 2006, 18, 2664–2679 CrossRef CAS PubMed.
- K. Lächler, J. Imhof, M. Reichelt, J. Gershenzon and S. Binder, The cytosolic branched-chain aminotransferases of Arabidopsis thaliana influence methionine supply, salvage and glucosinolate metabolism, Plant Mol. Biol., 2015, 88, 119–131 CrossRef PubMed.
- S.-G. Yu and L. O. Björn, Effects of UV-B radiation on light-dependent and light-independent protein phosphorylation in thylakoid membranes, J. Photochem. Photobiol., B, 1997, 37, 212–218 CrossRef CAS.
- I. Kalbina, S. Li, G. Kalbin, L. O. Björn and Å. Strid, Two separate UV-B radiation wavelength regions control expression of different molecular markers in Arabidopsis thaliana, Funct. Plant Biol., 2008, 35, 222–227 CrossRef CAS.
- U. Schreiber, U. Schliwa and W. Bilger, Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer, Photosynth. Res., 1986, 10, 51–62 CrossRef CAS PubMed.
- I. Šnyrychová and É. Hideg, The first application of terephthalate fluorescence for highly selective detection of hydroxyl radicals in thylakoid membranes, Funct. Plant Biol., 2007, 34, 1105–1111 CrossRef.
- S. Stoyanova, J. Geuns, É. Hideg and W. Van den Ende, The food additives inulin and stevioside counteract oxidative stress, Int. J. Food Sci. Nutr., 2012, 62, 207–214 CrossRef PubMed.
- S. Schmidt, et al., Identification of complex, naturally occuring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode array detection/electrospray ionization multi-stage mass spectrometry, Rapid Commun. Mass Spectrom., 2010, 24, 2009–2022 CrossRef CAS PubMed.
- S. Neugart, S. Rohn and M. Schreiner, Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile, Food Res. Int., 2015, 76, 114–121 CrossRef CAS.
- M. Wiesner, R. Zrenner, A. Krumbein, H. Glatt and M. Schreiner, Genotypic variation of the glucosinolate profile in pak choi (Brassica rapa ssp. chinensis), J. Agric. Food Chem., 2013, 61, 1943–1953 CrossRef CAS PubMed.
- Ø. Hammer, D. A. T. Harper and P. D. Ryan, PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 2001, 4, 1–9 Search PubMed.
- S. A. Denslow, A. A. Walls and M. E. Daub, Regulation of biosynthetic genes and antioxidant properties of Vitamin B6 vitamers during plant defense responses, Physiol. Mol. Plant Pathol., 2005, 66, 244–255 CrossRef CAS.
- P. Skorna, J. Rimarcik, P. Polia, V. Lukes and E. Klein, Thermodynamic study of vitamin B6 antioxidant potential, Comput. Theor. Chem., 2016, 1077, 32–38 CrossRef CAS.
- L. O. Morales, M. Brosché, J. Vainonen, G. I. Jenkins, J. Wargent, N. Sipari, Å. Strid, A. Lindfors, R. Tegelberg and P. J. Aphalo, Multiple roles for the UV RESISTANT LOCUS 8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar UV radiation, Plant Physiol., 2013, 161, 744–759 CrossRef CAS PubMed.
- É. Hideg, M. A. K. Jansen and Å. Strid, UV-B radiation, ROS and stress; inseparable companions or loosely linked associates?, Trends Plant Sci., 2013, 18, 107–115 CrossRef PubMed.
- M. Colinas, M. Eisenhut, T. Tohge, M. Pesquera, A. R. Fernie, A. P. M. Weber and T. B. Fitzpatrick, Balancing of B6 vitamers is essential for plant development and metabolism in Arabidopsis, Plant Cell, 2016, 28, 439–453 CrossRef CAS PubMed.
- P. Zimmermann, C. Heinlein, G. Orendi and U. Zentgraf, Senescence specific regulation of catalases in Arabidopsis thaliana (L.) Heynh, Plant, Cell Environ., 2006, 29, 1049–1060 CrossRef CAS PubMed.
- S. Bieker, L. Riester, M. Stahl, J. Franzaring and U. Zentgraf, Senescence-specific Alteration of Hydrogen Peroxide Levels in Arabidopsis thaliana and Oilseed Rape Spring Variety Brassica napus L. cv. Mozart, J. Integr. Plant Biol., 2012, 54, 540–554 CrossRef CAS PubMed.
- M. Heinze, F. S. Hanschen, M. Wiesner-Reinhold, S. Baldermann, J. Gräfe, M. Schreiner and S. Neugart, Effects of developmental stages and reduced UVB and low UV conditions on plant secondary metabolite profiles in Pak Choi (Brassica rapa subsp. chinensis), J. Agric. Food Chem., 2018, 66, 1678–1692 CrossRef CAS PubMed.
- J. J. Sheahan, Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae), Am. J. Bot., 1996, 83, 679–686 CrossRef CAS.
- S. Neugart and M. Schreiner, UVB and UVA as eustressors in horticultural and agricultural crops, Sci. Hortic., 2018, 234, 370–381 CrossRef CAS.
- P. V. Demkura and C. L. Ballaré, UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation, Mol. Plant, 2012, 5, 642–652 CrossRef PubMed.
- M. Götz, A. Albert, S. Stich, W. Heller, H. Scherb, A. Krins, C. Langebartels, H. K. Seidlitz and D. Ernst, PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period, Protoplasma, 2010, 243, 95–103 CrossRef PubMed.
- S. Neugart, M. Fiol, M. Schreiner, S. Rohn, R. Zrenner, L. W. Kroh and A. Krumbein, Interaction of moderate UV-B exposure and temperature on the formation of structurally different flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica), J. Agric. Food Chem., 2014, 62, 4054–4062 CrossRef CAS PubMed.
- B. Harbaum-Piayda, B. Walter, G. B. Bengtsson, E. M. Hubbermann, W. Bilger and K. Schwarz, Influence of pre-harvest UV-B irradiation and normal or controlled atmosphere storage on flavonoid and hydroxycinnamic acid contents of pak choi (Brassica campestris L. ssp chinensis var. communis), Postharvest Biol. Technol., 2010, 56, 202–208 CrossRef CAS.
- O. Rechner, S. Neugart, M. Schreiner, S. Wu and H. M. Poehling, Different narrow-band light ranges alter plant secondary metabolism and plant defense response to aphids, J. Chem. Ecol., 2016, 42, 989–1003 CrossRef CAS PubMed.
- M. Schreiner, et al., UV-B induced changes in secondary plant metabolites, in The role of UV-B radiation in plant growth and development, ed. B. R. Jordan, CABI Press, Oxford, UK, 2017, pp. 39–57. ISBN 978 1 78064 859 0 Search PubMed.
- Y. Wang, et al., Glucosinolate content and related gene expression in response to enhanced UV-B radiation in Arabidopsis, Afr. J. Biotechnol., 2011, 10, 6481–6491 CAS.
- I. Mewis, et al., UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors, Plant Cell Physiol., 2012, 53, 1546–1560 CrossRef CAS PubMed.
- M. F. Taviano, A. Melchini, A. Filocamo, C. Costa, S. Catania, R. Raciti, S. Saha, P. Needs, G. G. Bisignano and N. Miceli, Contribution of the glucosinolate fraction to the overall antioxidant potential, cytoprotection against oxidative insult and antimicrobial activity of Eruca sativa Mill. leaves extract, Pharmacogn. Mag., 2017, 13, 738–743 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00342h |
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |
