The unimolecular dissociation of magnesium chloride squarate (ClMgC4O4−) and reductive cyclooligomerisation of CO on magnesium†
Abstract
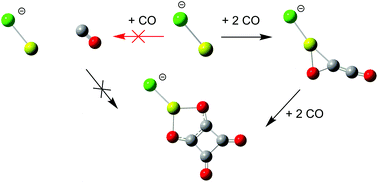
In this paper, we present an investigation of the unimolecular dissociation of an anionic magnesium chloride squarate complex, ClMgC4O4− using mass spectrometry supported by theoretical reaction models based on quantum chemical calculations. Sequential decarbonylation is the main fragmentation pathway leading to the deltate and ethenedione complexes, ClMgC3O3− and ClMgC2O2−, and MgCl−—yet the monomer, ClMgCO−, is not observed. Calculations using the G4 composite method show that the latter is unstable with respect to further dissociation. The implications for the reverse reaction sequence, cyclooligomerisation of CO on MgCl−, are discussed in detail and also compared with recent results from synthetic efforts in finding benign and efficient metal catalysed pathways to squaric acid from CO by reduction. It appears that the first step in these reactions, the formation of the first C–C bond by coupling of two CO molecules on MgCl−, is the most critical. The role of electron transfer in step-by-step stabilising the nascent CnOn centre is highlighted.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...