Synthetic feasibility of oxygen-driven photoisomerizations of alkenes and polyenes
Abstract
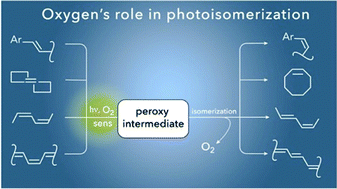
This review describes O2-dependent photoreactions for possible routes to double-bond isomerizations. E,Z-isomerizations triggered by O2 and visible light are a new area of potential synthetic interest. The reaction involves the reversible addition of O2 to form a peroxy intermediate with oxygen evolution and partial regeneration of the compound as its isomer. Targeting of O2-dependent photoisomerizations also relates to a practical use of visible light, for example the improved light penetration depth for visible as opposed to UV photons in batch sensitized reactions. This review is intended to draw a link between visible-light formation of a peroxy intermediate and its dark degradation with O2 release for unsaturated compound isomerization. This review should be of interest both to photochemists and synthetic organic chemists, as it ties together mechanistic and synthetic work, drawing attention to an overlooked subject.


 Please wait while we load your content...
Please wait while we load your content...
