Structural elucidation, total synthesis, and cytotoxic activity of effphenol A†
Abstract
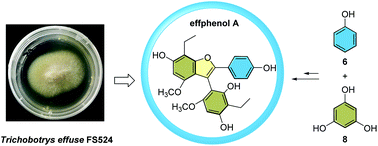
A highly substituted phenol derivative, effphenol A (1), was isolated from the deep-sea-derived fungus Trichobotrys effuse FS524. Its complete structural assignment was established through a combination of spectroscopic analysis together with single-crystal X-ray diffraction experiments and further unequivocally confirmed by a biomimetic total synthesis. Structurally, effphenol A possesses a poly-substituted 6-5/6/6 tetracyclic ring system, which represents the first case of such a skeleton found in nature. Furthermore, the cytotoxic activity of effphenol A (1) toward four human cancer cell lines was assayed.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...