Greener liquid-phase synthesis and the ACE inhibitory structure–activity relationship of an anti-SARS octapeptide†
Abstract
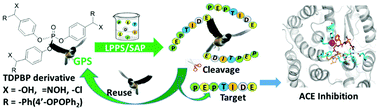
A high-efficiency strategy for resin-free and large scale liquid phase synthesis of the anti-SARS octapeptide AVLQSGFR is described. Herein, tri(4′-diphenylphosphonyloxylbenzoyl phenyl)phosphate (TDPBP) derivatives were designed as C-terminal supports to aid octapeptide intermediate purification without the need for chromatographic separation. Furthermore, the ACE inhibitory structure–activity relationship (SAR) of the anti-SARS octapeptide and its alanine-scanning analogues was systematically studied by in vitro assay and 3D-QSAR via molecular docking. This paper provides a new strategy for the development of peptide-based drugs. Simultaneously, a study on the ACE inhibition and structure–activity relationship of the anti-SARS octapeptide also lays a foundation for further understanding how the anti-SARS octapeptide acts as an ACE inhibitor.
- This article is part of the themed collections: Coronavirus articles - free to access collection and Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...