Synthesis and antiproliferative effect of the proposed stereoisomer of the marine sponge metabolite halisphingosine A†
Abstract
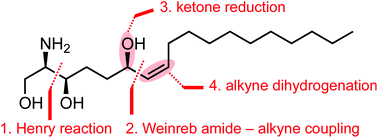
The first synthesis of the proposed natural stereoisomer of halisphingosine A, a metabolite of the marine sponge Haliclona tubifera, was accomplished in 11 steps including an enantioselective Henry reaction, a Weinreb amide – acetylide coupling, and stereoselective reductions of the resulting ynone to afford the R,Z-configured allyl alcohol moiety. The synthetic product differed from the natural isolate in some 13C-NMR data. It showed antiproliferative activity at clinically relevant concentrations against six tumour cell lines including such lacking functional tumor suppressor gene p53.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...