Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnesium-specific ring expansion/contraction catalysed by the class II diterpene cyclase from pleuromutilin biosynthesis†
Cody
Lemke
,
Owen
Whitham
and
Reuben J.
Peters
 *
*
Roy J. Carver Department of Biochemistry, Biophysics & Molecular Biology, Iowa State University, Ames, IA 50011, USA. E-mail: rjpeters@iastate.edu
First published on 10th July 2020
Abstract
The class II diterpene cyclase (DTC) from pleuromutilin biosynthesis uniquely mediates ‘A’ ring contraction of the initially formed decalin bicycle, yielding mutildienyl diphosphate (MPP). Catalysis requires a divalent metal cation co-factor. Intriguingly, selectively with magnesium, this DTC catalyzes ring expansion/contraction between MPP and halimadienyl diphosphate, providing some catalytic insight.
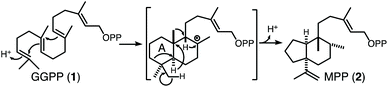
Pleuromutilin is a fungal diterpenoid natural product that serves as a precursor to pharmaceutically relevant antibiotics.1 Production of the underlying tricyclic “propellane-type” hydrocarbon backbone is catalysed by a bifunctional enzyme representing fusion of a sequentially acting class II diterpene cyclase (DTC) and class I diterpene synthase, termed a premutilin synthase (PS) after the resulting diterpene.2,3 Previous work with the relevant enzyme from Clitopilus passeckeranis (CpPS) demonstrated that leucine substitution for aspartate 649 blocks class I activity, enabling study of the DTC activity in isolation. In particular, metabolic engineering, via heterologous expression of this CpPS:D649L mutant in Escherichia coli also engineered to produce the general diterpene precursor [E,E,E]-geranylgeranyl diphosphate (GGPP, 1), allowed identification of the relevant DTC product as mutildienyl diphosphate (MPP, 2).4 Notably, formation of 2 requires ring contraction of the initially formed decalin bicycle, which seems to be unique to this DTC.5 More specifically, contraction of the ‘A’ ring, requiring a preceding series of 1,2-shifts (Scheme 1).
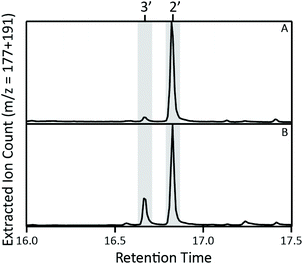
Intriguingly, while after induction these recombinant cultures initially only produce 2 (observed as mutildien-15-ol, 2′, derived from dephosphorylation of 2 catalysed by endogenous phosphatases),4 with sufficient time (>1 day) another DTC product was observed in slowly increasing amounts (Fig. 1 and S1†). Comparison to authentic standards readily identified this as syn-halima-5,13E-dienyl diphosphate (HPP, 3, also observed as the dephosphorylated derivative – i.e., syn-halimadien-15-ol, 3′). Notably, formation of 3 represents deprotonation of the penultimate intermediate, syn-halima-13E-15-PP-5-yl+ (3+), just before the unique ring contraction step, in the reaction leading to 2. However, the delayed appearance of 3 suggests that it might be produced from 2, which would represent ‘A’ ring expansion, essentially reversing the final ring contraction step in the reaction catalysed from 1.
This surprising production of 3 by CpPS:D649L was first investigated by co-expression experiments using the D311A mutant of CpPS that blocks DTC activity.4 Notably, whereas co-expression of CpPS:D311A with CpPS:D649L in E. coli also engineered to produce 1 led to production of premutilin (4) with no detection of 3, analogous co-expression of a DTC that produces 3 (OsCPS4:H501D)6 with CpPS:D311A did not. Instead, as identified by comparison to an authentic standard,7 isotuberculosinol/nosyberkol (5) was observed (Fig. S2†). This is derived from addition of water to the tertiary carbocation formed by lysis of the allylic diphosphate ester bond in 3, consistent with the known class I activity of CpPS (i.e., the hydroxyl group found in premutilin). Thus, 3 is not a precursor to 4 and, accordingly, CpPS:D649L does not seem to produce 3 directly from 1 (i.e., as 3 is not detected upon co-expression with CpPS:D311A). This suggests that the CpPS DTC active site seems be able to produce 3 from its usual product 2, albeit this ring expansion reaction is clearly much less efficient than that catalysed by its class I active site (i.e., to produce 4).
To further investigate the hypothesis that the CpPS DTC active site is capable of catalysing this intriguing partial reverse (ring expansion) reaction in vitro assays were carried out. Consistent with the production of 3 from 2, accumulation of 3 was delayed relative to 2 (Fig. S3†). Notably, in the course of optimizing conditions for the in vitro assays it was found that, while altering pH and buffer or salt did not significantly alter production of 3 (Fig. S4†) increasing the concentration of the magnesium (Mg2+) co-factor led to higher rates of production (Fig. S5†). By contrast, assays in the presence of a variety of alternative divalent metal ions (0.1 mM Ca2+, Co2+ or Ni2+) all produced only 2, with no 3 observed (Fig. S6†). Moreover, further transformation of 2 into 3 required active CpPS as well as Mg2+ (Fig. S7†). These results indicate that CpPS specifically can use Mg2+ to catalyse ring expansion of 2 to produce 3, albeit at a much slower rate than the production of 2 from 1. Nevertheless, this finding enabled separate examination of the production of 2versus both this and 3.
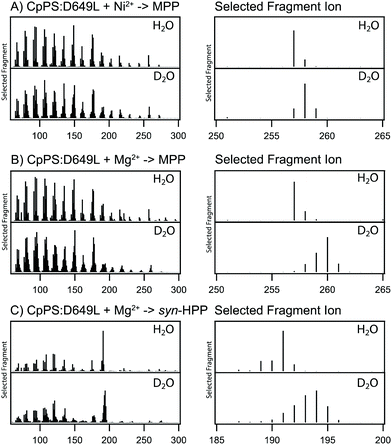
The production of 3 from 2 requires an additional protonation step beyond that necessitated by the formation of 2 from 1 (see Scheme 2). This mechanistic implication was investigated by deuterium labelling studies. Specifically, assays carried out in 2H2O (D2O) versus H2O. As expected, when CpPS was limited to the production of just 2 (by use of Ni2+), in D2O the resulting 2 contained a single deuterium (Fig. 2A). Intriguingly, in the presence of Mg2+ the resulting 2 and 3 were both multiply labelled as evidenced by increases in the m/z of the observed fragments (Fig. 2B and C). Accordingly, not only does this provide strong support for the production of 3 from 2 rather than directly from 1, it further indicates that, selectively in the presence of Mg2+, the CpPS DTC active site can catalyse interconversion of 2 and 3. Note that the observed essentially equivalent labelling is consistent with the previously demonstrated (methyl) specific deprotonation that yields 2,8,9 as well as expected stereospecific protonation/deprotonation at C6 of the endo olefin in 3 (Scheme S1†).
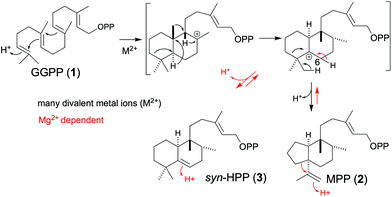 | ||
| Scheme 2 CpPS DTC catalysed reactions from 1 to 2, and (inefficient) ring expansion/contraction interconverting 2 and 3. | ||
Conclusions
Interconversion of 2 with 3 requires protonation of the isopropylene moiety in 2 or endo-cyclic alkene in 3 (Scheme 2). While mechanistically analogous to the protonation of 1 catalysed by all DTCs, there is a clear difference in location of the targeted olefins. Given the greasy nature of the hydrocarbon portion of the substrate in each of these cases, binding is presumably dominated by the diphosphate moiety. In turn, this is positioned by interactions with the divalent metal ion co-factor(s).10 Accordingly, the Mg2+-dependent nature of the observed ring expansion/contraction reactions indicates that only this divalent metal ion correctly positions 2 and 3 for protonation. Moreover, this configuration must further enable their interconversion by leaving the resulting ring expanded or contracted intermediates appropriately positioned for deprotonation by a catalytic base. Thus, while the use of alternative divalent metal ions has been shown to affect DTC catalytic efficiency,11 the results reported here demonstrate that these co-factors also can affect product outcome through effects on orientation of the substrate/reactant.Conflicts of interest
R. J. P. is a member of the scientific advisory board of Manus Bio, Inc.Acknowledgements
This work was supported by a grant from the NIH to R. J. P. (GM131885).References
- S. Paukner and R. Riedl, Cold Spring Harb. Perspect Med., 2017, 7, 1–15 CrossRef PubMed.
- F. Alberti, K. Khairudin, E. R. Venegas, J. A. Davies, P. M. Hayes, C. L. Willis, A. M. Bailey and G. D. Foster, Nat. Commun., 2017, 8, 1831 CrossRef.
- M. Yamane, A. Minami, C. Liu, T. Ozaki, I. Takeuchi, T. Tsukagoshi, T. Tokiwano, K. Gomi and H. Oikawa, ChemBioChem, 2017, 18, 2317–2322 CrossRef CAS.
- M. Xu, M. Jia, Y. J. Hong, X. Yin, D. J. Tantillo, P. J. Proteau and R. J. Peters, Org. Lett., 2018, 20, 1200–1202 CrossRef CAS.
- R. J. Peters, Nat. Prod. Rep., 2010, 27, 1521–1530 RSC.
- K. C. Potter, M. Jia, Y. J. Hong, D. J. Tantillo and R. J. Peters, Org. Lett., 2016, 18, 1060–1063 CrossRef CAS PubMed.
- M. Jia, K. C. Potter and R. J. Peters, Metab. Eng., 2016, 37, 24–34 CrossRef CAS PubMed.
- A. J. Birch, C. W. Hozapfel and R. W. Rickards, Tetrahedron, 1966, 22, 359–387 CrossRef.
- D. Arigoni, Pure Appl. Chem., 1968, 17, 331–348 CAS.
- Y. Gao, R. B. Honzatko and R. J. Peters, Nat. Prod. Rep., 2012, 29, 1153–1175 RSC.
- S. Prisic and R. J. Peters, Plant Physiol., 2007, 144, 445–454 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods and supplemental figures. See DOI: 10.1039/d0ob01422b |
| This journal is © The Royal Society of Chemistry 2020 |