Use of solid-supported 4-fluorophenyl 3-nitro-2-pyridinesulfenate in the construction of disulfide-linked hybrid molecules†
Abstract
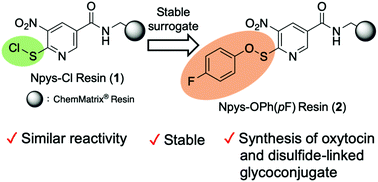
To construct disulfide-linked hybrid molecules systematically and efficiently, we established a more practical solid-phase disulfide ligation (SPDSL) system with enhanced utility. The group Npys-OPh(pF) shows reactivity similar to that of Npys-Cl, but it is more stable. An efficient synthesis of the cyclic peptide oxytocin and a peptide–sugar conjugate was accomplished as models. These results indicate that the Npys-OPh(pF) resin functions as a common synthetic platform in SPDSL.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...