l-Proline-catalyzed regioselective C1 arylation of tetrahydroisoquinolines through a multicomponent reaction under solvent-free conditions†
Abstract
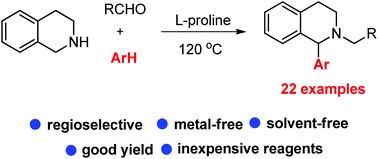
Here we disclose the C1 arylation of tetrahydroisoquinolines (THIQ) through regioselective C(sp3)–H functionalization using a multicomponent reaction. The reaction was performed by reacting THIQ, aldehydes and aminopyrazoles or indoles under neat conditions with L-proline as a catalyst. The regioselectivity of the products was confirmed by X-ray analysis and spectroscopic data. The formation of an azomethine ylide intermediate is crucial for obtaining the regioselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...