Monitoring ligand-mediated helix 12 transitions within the human estrogen receptor α using bipartite tetracysteine display†
Abstract
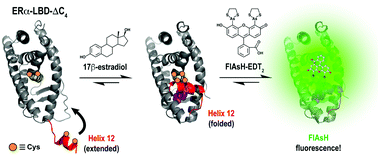
Estrogen receptor α ligand-binding domains (ERα-LBD) expressing tetracysteine motifs bind FlAsH-EDT2 upon transition of helix 12 (H12) to a folded state. Changes in fluorescence intensity allowed surveillance of ligand-mediated H12 transitions and facilitated the determination of FlAsH association rates (kon) and apparent equilibrium dissociation constants (Kapp) to ERα-LBDs in the presence of estrogenic ligands.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...